Inability of plasmacytoid dendritic cells to directly lyse HIV-infected autologous CD4+ T cells despite induction of tumor necrosis factor-related apoptosis-inducing ligand
- PMID: 20042498
- PMCID: PMC2826047
- DOI: 10.1128/JVI.01350-09
Inability of plasmacytoid dendritic cells to directly lyse HIV-infected autologous CD4+ T cells despite induction of tumor necrosis factor-related apoptosis-inducing ligand
Abstract
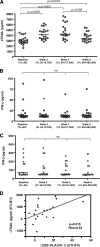
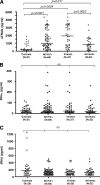
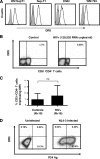
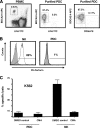
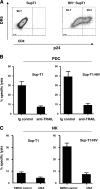
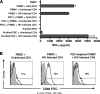
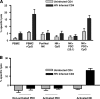
The function of plasmacytoid dendritic cells (PDC) in chronic human immunodeficiency virus type 1 (HIV-1) infection remains controversial with regard to its potential for sustained alpha interferon (IFN-alpha) production and induction of PDC-dependent tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL)-mediated cytotoxicity of HIV-infected cells. We address these areas by a study of chronically HIV-1-infected subjects followed through antiretroviral therapy (ART) interruption and by testing PDC cytolytic function against autologous HIV-infected CD4(+) T cells. Rebound in viremia induced by therapy interruption showed a positive association between TRAIL and viral load or T-cell activation, but comparable levels of plasma IFN-alpha/beta were found in viremic ART-treated and control subjects. While PDC from HIV-infected subjects expressed less interferon regulator factor 7 (IRF-7) and produced significantly less IFN-alpha upon Toll-like receptor 7/9 (TLR7/9) engagement than controls, membrane TRAIL expression in PDC from HIV(+) subjects was increased. Moreover, no significant increase in death receptor 5 (DR5) expression was seen in CD4(+) T cells from viremic HIV(+) subjects compared to controls or following in vitro infection/exposure to infectious and noninfectious virus or exogenous IFN-alpha, respectively. Although activated PDC killed the DR5-expressing HIV-infected Sup-T1 cell line, PDC did not lyse primary autologous HIV(+) CD4(+) T cells yet could provide accessory help for NK cells in killing HIV-infected autologous CD4(+) T cells. Taken together, our data show a lack of sustained high levels of soluble IFN-alpha in chronic HIV-1 infection in vivo and document a lack of direct PDC cytolytic activity against autologous infected or uninfected CD4(+) T cells.
Figures


Similar articles
-
Plasmacytoid dendritic cells express TRAIL and induce CD4+ T-cell apoptosis in HIV-1 viremic patients.Blood. 2009 Oct 29;114(18):3854-63. doi: 10.1182/blood-2009-04-217927. Epub 2009 Aug 18. Blood. 2009. PMID: 19690337
-
HMGB1 Is Involved in IFN-α Production and TRAIL Expression by HIV-1-Exposed Plasmacytoid Dendritic Cells: Impact of the Crosstalk with NK Cells.PLoS Pathog. 2016 Feb 12;12(2):e1005407. doi: 10.1371/journal.ppat.1005407. eCollection 2016 Feb. PLoS Pathog. 2016. PMID: 26871575 Free PMC article.
-
Are blockers of gp120/CD4 interaction effective inhibitors of HIV-1 immunopathogenesis?AIDS Rev. 2006 Jan-Mar;8(1):3-8. AIDS Rev. 2006. PMID: 16736946 Review.
-
Plasmacytoid dendritic cells (pDCs) from HIV controllers produce interferon-α and differentiate into functional killer pDCs under HIV activation.J Infect Dis. 2012 Sep 1;206(5):790-801. doi: 10.1093/infdis/jis384. Epub 2012 Jun 12. J Infect Dis. 2012. PMID: 22693234
-
HIV-1 immunopathogenesis: how good interferon turns bad.Clin Immunol. 2007 May;123(2):121-8. doi: 10.1016/j.clim.2006.09.016. Epub 2006 Nov 16. Clin Immunol. 2007. PMID: 17112786 Free PMC article. Review.
Cited by
-
Plasmacytoid dendritic cells suppress HIV-1 replication but contribute to HIV-1 induced immunopathogenesis in humanized mice.PLoS Pathog. 2014 Jul 31;10(7):e1004291. doi: 10.1371/journal.ppat.1004291. eCollection 2014 Jul. PLoS Pathog. 2014. PMID: 25077616 Free PMC article.
-
Dendritic Cells in HIV-1 and HCV Infection: Can They Help Win the Battle?Virology (Auckl). 2013 Feb 11;4:1-25. doi: 10.4137/VRT.S11046. eCollection 2013. Virology (Auckl). 2013. PMID: 25512691 Free PMC article. Review.
-
Type I Interferon at the Interface of Antiviral Immunity and Immune Regulation: The Curious Case of HIV-1.Scientifica (Cairo). 2013;2013:580968. doi: 10.1155/2013/580968. Epub 2013 Dec 22. Scientifica (Cairo). 2013. PMID: 24455433 Free PMC article. Review.
-
HIV integrase and the swan song of the CD4 T cells?Retrovirology. 2013 Dec 9;10:149. doi: 10.1186/1742-4690-10-149. Retrovirology. 2013. PMID: 24321528 Free PMC article.
-
Plasmacytoid dendritic cell number and responses to Toll-like receptor 7 and 9 agonists vary in HIV Type 1-infected individuals in relation to clinical state.AIDS Res Hum Retroviruses. 2013 Mar;29(3):501-10. doi: 10.1089/aid.2012.0200. Epub 2013 Jan 15. AIDS Res Hum Retroviruses. 2013. PMID: 23131038 Free PMC article.
References
-
- Audige, A., M. Urosevic, E. Schlaepfer, R. Walker, D. Powell, S. Hallenberger, H. Joller, H. U. Simon, R. Dummer, and R. F. Speck. 2006. Anti-HIV state but not apoptosis depends on IFN signature in CD4+ T cells. J. Immunol. 177:6227-6237. - PubMed
-
- Beignon, A. S., K. McKenna, M. Skoberne, O. Manches, I. DaSilva, D. G. Kavanagh, M. Larsson, R. J. Gorelick, J. D. Lifson, and N. Bhardwaj. 2005. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J. Clin. Investig. 115:3265-3275. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

