Interaction domains of the UL16 and UL21 tegument proteins of herpes simplex virus
- PMID: 20042500
- PMCID: PMC2826038
- DOI: 10.1128/JVI.02015-09
Interaction domains of the UL16 and UL21 tegument proteins of herpes simplex virus
Abstract
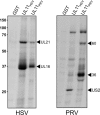
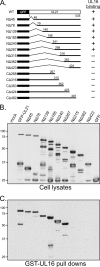
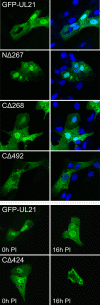
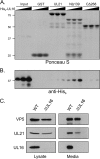
The UL16 protein of herpes simplex virus is capsid associated and was previously identified as a binding partner of the membrane-associated UL11 tegument protein (J. S. Loomis, R. J. Courtney, and J. W. Wills, J. Virol. 77:11417-11424, 2003). In those studies, a less-prominent, approximately 65-kDa binding partner of unknown identity was also observed. Mass spectrometry studies have now revealed this species to be UL21, a tegument protein that has been implicated in the transport of capsids in the cytoplasm. The validity of the mass spectrometry results was tested in a variety of coimmunoprecipitation and glutathione S-transferase pull-down experiments. The data revealed that UL21 and UL16 can form a complex in the absence of other viral proteins, even when the assays used proteins purified from Escherichia coli. Moreover, UL11 was able to pull down UL21 only when UL16 was present, suggesting that all three proteins can form a complex. Deletion analyses revealed that the second half of UL21 (residues 268 to 535) is sufficient for the UL16 interaction and packaging into virions; however, attempts to map a subdomain of UL16 were largely unsuccessful, with only the first 40 (of 373) residues being found to be dispensable. Nevertheless, it is clear that UL16 must have two distinct binding sites, because covalent modification of its free cysteines with N-ethylmaleimide blocked binding to UL11 but not UL21. These findings should prove useful for elucidating the molecular machinery used to transmit a signal into a virion when it attaches to cells, a recently discovered mechanism in which UL16 is a central player.
Figures




References
-
- Addison, C., F. J. Rixon, and V. G. Preston. 1990. Herpes simplex virus type 1 UL28 gene product is important for the formation of mature capsids. J. Gen. Virol. 71:2377-2384. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

