Serotype-specific structural differences in the protease-cofactor complexes of the dengue virus family
- PMID: 20042502
- PMCID: PMC2826037
- DOI: 10.1128/JVI.02044-09
Serotype-specific structural differences in the protease-cofactor complexes of the dengue virus family
Abstract
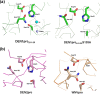
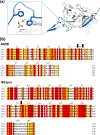
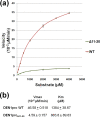
With an estimated 40% of the world population at risk, dengue poses a significant threat to human health, especially in tropical and subtropical regions. Preventative and curative efforts, such as vaccine development and drug discovery, face additional challenges due to the occurrence of four antigenically distinct serotypes of the causative dengue virus (DEN1 to -4). Complex immune responses resulting from repeat assaults by the different serotypes necessitate simultaneous targeting of all forms of the virus. One of the promising targets for drug development is the highly conserved two-component viral protease NS2B-NS3, which plays an essential role in viral replication by processing the viral precursor polyprotein into functional proteins. In this paper, we report the 2.1-A crystal structure of the DEN1 NS2B hydrophilic core (residues 49 to 95) in complex with the NS3 protease domain (residues 1 to 186) carrying an internal deletion in the N terminus (residues 11 to 20). While the overall folds within the protease core are similar to those of DEN2 and DEN4 proteases, the conformation of the cofactor NS2B is dramatically different from those of other flaviviral apoprotease structures. The differences are especially apparent within its C-terminal region, implicated in substrate binding. The structure reveals for the first time serotype-specific structural elements in the dengue virus family, with the reported alternate conformation resulting from a unique metal-binding site within the DEN1 sequence. We also report the identification of a 10-residue stretch within NS3pro that separates the substrate-binding function from the catalytic turnover rate of the enzyme. Implications for broad-spectrum drug discovery are discussed.
Figures



References
-
- Adams, P. D., R. W. Grosse-Kunstleve, L. W. Hung, T. R. Ioerger, A. J. McCoy, N. W. Moriarty, R. J. Read, J. C. Sacchettini, N. K. Sauter, and T. C. Terwilliger. 2002. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr. D Biol. Crystallogr. 58:1948-1954. - PubMed
-
- Collaborative Computational Project Number 4. 1994. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 50:760-763. - PubMed
-
- De Clercq, E. 2007. The design of drugs for HIV and HCV. Nat. Rev. Drug Discov. 6:1001-1018. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

