Activation of peroxisome proliferator-activated receptor gamma by human cytomegalovirus for de novo replication impairs migration and invasiveness of cytotrophoblasts from early placentas
- PMID: 20042507
- PMCID: PMC2826069
- DOI: 10.1128/JVI.01779-09
Activation of peroxisome proliferator-activated receptor gamma by human cytomegalovirus for de novo replication impairs migration and invasiveness of cytotrophoblasts from early placentas
Abstract
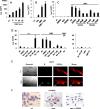
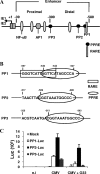
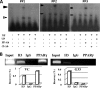
Human cytomegalovirus (HCMV) contributes to pathogenic processes in immunosuppressed individuals, in fetuses, and in neonates. In the present report, by using reporter gene activation assays and confocal microscopy in the presence of a specific antagonist, we show for the first time that HCMV infection induces peroxisome proliferator-activated receptor gamma (PPARgamma) transcriptional activity in infected cells. We demonstrate that the PPARgamma antagonist dramatically impairs virus production and that the major immediate-early promoter contains PPAR response elements able to bind PPARgamma, as assessed by electrophoretic mobility shift and chromatin immunoprecipitation assays. Due to the key role of PPARgamma in placentation and its specific trophoblast expression within the human placenta, we then provided evidence that by activating PPARgamma human cytomegalovirus dramatically impaired early human trophoblast migration and invasiveness, as assessed by using well-established in vitro models of invasive trophoblast, i.e., primary cultures of extravillous cytotrophoblasts (EVCT) isolated from first-trimester placentas and the EVCT-derived cell line HIPEC. Our data provide new clues to explain how early infection during pregnancy could impair implantation and placentation and therefore embryonic development.
Figures




Similar articles
-
PPARγ and human trophoblast differentiation.J Reprod Immunol. 2011 Jun;90(1):41-9. doi: 10.1016/j.jri.2011.05.003. Epub 2011 Jun 24. J Reprod Immunol. 2011. PMID: 21704384
-
Cytomegalovirus Infection Triggers the Secretion of the PPARγ Agonists 15-Hydroxyeicosatetraenoic Acid (15-HETE) and 13-Hydroxyoctadecadienoic Acid (13-HODE) in Human Cytotrophoblasts and Placental Cultures.PLoS One. 2015 Jul 14;10(7):e0132627. doi: 10.1371/journal.pone.0132627. eCollection 2015. PLoS One. 2015. PMID: 26171612 Free PMC article.
-
PPARgamma and early human placental development.Curr Med Chem. 2008;15(28):3011-24. doi: 10.2174/092986708786848677. Curr Med Chem. 2008. PMID: 19075649 Review.
-
Expression and role of peroxisome proliferator-activated receptors in the porcine early placenta trophoblast.Domest Anim Endocrinol. 2019 Apr;67:42-53. doi: 10.1016/j.domaniend.2018.12.001. Epub 2018 Dec 11. Domest Anim Endocrinol. 2019. PMID: 30690257
-
Role of nuclear receptors and their ligands in human trophoblast invasion.J Reprod Immunol. 2008 Apr;77(2):161-70. doi: 10.1016/j.jri.2007.05.004. Epub 2007 Aug 15. J Reprod Immunol. 2008. PMID: 17706792 Review.
Cited by
-
Pregnancy and viral infections: Mechanisms of fetal damage, diagnosis and prevention of neonatal adverse outcomes from cytomegalovirus to SARS-CoV-2 and Zika virus.Biochim Biophys Acta Mol Basis Dis. 2021 Oct 1;1867(10):166198. doi: 10.1016/j.bbadis.2021.166198. Epub 2021 Jun 10. Biochim Biophys Acta Mol Basis Dis. 2021. PMID: 34118406 Free PMC article. Review.
-
Human placental trophoblast invasion and differentiation: a particular focus on Wnt signaling.Front Genet. 2013 Sep 26;4:190. doi: 10.3389/fgene.2013.00190. Front Genet. 2013. PMID: 24133501 Free PMC article. Review.
-
Vaccine-Derived Neutralizing Antibodies to the Human Cytomegalovirus gH/gL Pentamer Potently Block Primary Cytotrophoblast Infection.J Virol. 2015 Dec;89(23):11884-98. doi: 10.1128/JVI.01701-15. Epub 2015 Sep 16. J Virol. 2015. PMID: 26378171 Free PMC article.
-
Role of human cytomegalovirus in the proliferation and invasion of extravillous cytotrophoblasts isolated from early placentae.Int J Clin Exp Med. 2015 Oct 15;8(10):17248-60. eCollection 2015. Int J Clin Exp Med. 2015. PMID: 26770317 Free PMC article.
-
Human Cytomegalovirus Infection Changes the Pattern of Surface Markers of Small Extracellular Vesicles Isolated From First Trimester Placental Long-Term Histocultures.Front Cell Dev Biol. 2021 Sep 10;9:689122. doi: 10.3389/fcell.2021.689122. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34568315 Free PMC article.
References
-
- AbuBakar, S., I. Boldogh, and T. Albrecht. 1990. Human cytomegalovirus stimulates arachidonic acid metabolism through pathways that are affected by inhibitors of phospholipase A2 and protein kinase C. Biochem. Biophys. Res. Commun. 166:953-959. - PubMed
-
- Barak, Y., M. C. Nelson, E. S. Ong, Y. Z. Jones, P. Ruiz-Lozano, K. R. Chien, A. Koder, and R. M. Evans. 1999. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol. Cell 4:585-595. - PubMed
-
- Collet, P., L. Domenjoud, M. D. Devignes, H. Murad, H. Schohn, and M. Dauca. 2004. The human semaphorin 6B gene is down regulated by PPARs. Genomics 83:1141-1150. - PubMed
-
- Desvergne, B., and W. Wahli. 1999. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr. Rev. 20:649-688. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

