Immunogenicity of a heptavalent conjugate pneumococcal vaccine administered concurrently with a combination diphtheria, tetanus, five-component acellular pertussis, inactivated polio, and Haemophilus influenzae type B vaccine and a meningococcal group C conjugate vaccine at 2, 3, and 4 months of age
- PMID: 20042517
- PMCID: PMC2837965
- DOI: 10.1128/CVI.00315-09
Immunogenicity of a heptavalent conjugate pneumococcal vaccine administered concurrently with a combination diphtheria, tetanus, five-component acellular pertussis, inactivated polio, and Haemophilus influenzae type B vaccine and a meningococcal group C conjugate vaccine at 2, 3, and 4 months of age
Abstract
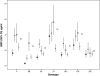
The immunogenicities of conjugate pneumococcal vaccines have been demonstrated when they are administered at 2, 3, and 4 months of age. There is a paucity of data on the immunogenicity of this vaccine when it is administered concurrently with other vaccines in the primary immunization schedule of the United Kingdom. We immunized 55 term infants at 2, 3, and 4 months of age with the seven-valent pneumococcal conjugate vaccine (PCV7), the meningococcal group C conjugate (MCC) vaccine, and the diphtheria, tetanus, five-component acellular pertussis, inactivated polio, and Haemophilus influenzae type b (DTaP(5)/IPV/Hib-TT) vaccine. The immune responses to the H. influenzae type b (Hib), MCC, and tetanus vaccines were measured at 2, 5, and 12 months of age; and the immune responses to PCV7 were measured at 2 and 5 months and then either at 12 months or following a 4th dose of PCV7. There were increases in the geometric mean concentrations (GMCs) of all antigens postimmunization. Greater than or equal to 90% of the infants achieved putatively protective levels postimmunization for all vaccine antigens except pneumococcal serotype 6B and Hib. The GMCs of the PCV7 serotypes increased following a 4th dose, although one infant had not reached putative levels of protection against serotype 6B. In conclusion, when infants were vaccinated according to the schedule described above, they had lower postprimary immunization responses to Hib, meningococcus group C capsular polysaccharide, and pneumococcal serotype 6B than the responses demonstrated by use of the other schedules. Despite this finding, there was a good response following a 4th dose of PCV7.
Figures
References
-
- Berrington, J. E., A. J. Cant, J. N. Matthews, M. O'Keeffe, G. P. Spickett, and A. C. Fenton. 2006. Haemophilus influenzae type b immunization in infants in the United Kingdom: effects of diphtheria/tetanus/acellular pertussis/Hib combination vaccine, significant prematurity, and a fourth dose. Pediatrics 117:e717-e724. - PubMed
-
- Black, S., H. Shinefield, B. Fireman, E. Lewis, P. Ray, J. R. Hansen, L. Elvin, K. M. Ensor, J. Hackell, G. Siber, F. Malinoski, D. Madore, I. Chang, R. Kohberger, W. Watson, R. Austrian, K. Edwards, J. Aguilar, M. Bartlett, R. Bergen, M. Burman, S. Dorfman, W. Easter, A. Finkel, H. Froehlich, J. Glauber, A. Herz, D. Honeychurch, R. Kleinrock, I. Landaw, A. Lavetter, C. Le, S. McMurtry, P. Morozumi, P. Mullin, M. Rehbein, R. Rossin, G. Soe, I. Takahashi, G. Udkow, and R. Whitson. 2000. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr. Infect. Dis. J. 19:187-195. - PubMed
-
- Borrow, R., and E. B. Kaczmarksi. 2000. Meningococcal serology, p. 289-304. In A. J. Pollard and M. C. J. Maiden (ed.), Methods in molecular medicine. Human Press, Totowa, NJ.
-
- Buttery, J. P., A. Riddell, J. McVernon, T. Chantler, L. Lane, J. Bowen-Morris, L. Diggle, R. Morris, A. Harnden, S. Lockhart, A. J. Pollard, K. Cartwright, and E. R. Moxon. 2005. Immunogenicity and safety of a combination pneumococcal-meningococcal vaccine in infants: a randomized controlled trial. JAMA 293:1751-1758. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

