Functional deficiencies and a reduced response to calcium in the flagellum of mouse sperm lacking SPAG16L
- PMID: 20042536
- PMCID: PMC2842488
- DOI: 10.1095/biolreprod.109.080143
Functional deficiencies and a reduced response to calcium in the flagellum of mouse sperm lacking SPAG16L
Abstract
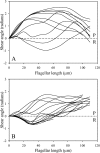
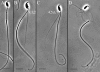
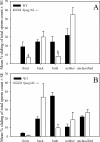
The Spag16L gene codes for a protein that is localized to the central apparatus which is essential for normal sperm motility and male fertility. Sperm from mice homozygous for a targeted deletion of the Spag16L gene were examined to assess their flagellar motor functions compared with age- and strain-matched control sperm. Sperm were also demembranated with Triton X-100 and examined for their ability to respond to free calcium, as well as for their ability to undergo microtubule sliding driven by dynein action. In addition, the passive flagella, inhibited by sodium metavanadate to disable the dyneins, were examined for mechanical abnormalities. Live Spag16L-null sperm exhibited much less bending of the flagellum during the beat. The amount of microtubule sliding in the R-bend direction of the beat was selectively restricted, which suggests that there is limited activation of the dyneins on one side of the axoneme in the live cells. This is corroborated by the results on detergent-extracted sperm models. The flagellar response to calcium is greatly reduced. The calcium response requires the activation of the dyneins on outer doublets 1, 2, 3, and 4. These are the same dyneins required for R-bend formation. In axonemes prepared to disintegrate by microtubule sliding, we observed little or no extrusion of doublets 1 and 2, consistent with a reduced activity of their dyneins. This deficit in motor function, and an increased rigidity of the midpiece region which we detected in the passive flagella, together can explain the observed motility characteristics of the Spag16L-null sperm.
Figures





Similar articles
-
Central-pair-linked regulation of microtubule sliding by calcium in flagellar axonemes.J Cell Sci. 2003 Apr 15;116(Pt 8):1627-36. doi: 10.1242/jcs.00336. J Cell Sci. 2003. PMID: 12640046
-
Sperm flagella: comparative and phylogenetic perspectives of protein components.Mol Hum Reprod. 2011 Aug;17(8):524-38. doi: 10.1093/molehr/gar034. Epub 2011 May 17. Mol Hum Reprod. 2011. PMID: 21586547 Review.
-
Evidence for an increased sensitivity to Ca2+ in the flagella of sperm from tw32/+mice.Mol Reprod Dev. 1990 May;26(1):69-77. doi: 10.1002/mrd.1080260111. Mol Reprod Dev. 1990. PMID: 2346648
-
The axonemal axis and Ca2+-induced asymmetry of active microtubule sliding in sea urchin sperm tails.J Cell Biol. 1986 Jun;102(6):2042-52. doi: 10.1083/jcb.102.6.2042. J Cell Biol. 1986. PMID: 2940250 Free PMC article.
-
Analysis of the sperm flagellar axoneme using gene-modified mice.Exp Anim. 2020 Nov 12;69(4):374-381. doi: 10.1538/expanim.20-0064. Epub 2020 Jun 18. Exp Anim. 2020. PMID: 32554934 Free PMC article. Review.
Cited by
-
The many modes of flagellar and ciliary beating: Insights from a physical analysis.Cytoskeleton (Hoboken). 2021 Feb;78(2):36-51. doi: 10.1002/cm.21656. Epub 2021 Mar 15. Cytoskeleton (Hoboken). 2021. PMID: 33675288 Free PMC article. Review.
-
Spag16, an axonemal central apparatus gene, encodes a male germ cell nuclear speckle protein that regulates SPAG16 mRNA expression.PLoS One. 2011;6(5):e20625. doi: 10.1371/journal.pone.0020625. Epub 2011 May 31. PLoS One. 2011. PMID: 21655194 Free PMC article.
-
Actin-related protein ACTL7B ablation leads to OAT with multiple morphological abnormalities of the flagellum and male infertility in mice†.Biol Reprod. 2023 Mar 13;108(3):447-464. doi: 10.1093/biolre/ioad001. Biol Reprod. 2023. PMID: 36617158 Free PMC article.
-
Mammalian axoneme central pair complex proteins: Broader roles revealed by gene knockout phenotypes.Cytoskeleton (Hoboken). 2016 Jan;73(1):3-22. doi: 10.1002/cm.21271. Cytoskeleton (Hoboken). 2016. PMID: 26785425 Free PMC article. Review.
-
Genetic variation in SPAG16 regions encoding the WD40 repeats is not associated with reduced sperm motility and axonemal defects in a population of infertile males.BMC Urol. 2012 Sep 10;12:27. doi: 10.1186/1471-2490-12-27. BMC Urol. 2012. PMID: 22963137 Free PMC article.
References
-
- Zhang Z, Sapiro R, Kapfhamer D, Bucan M, Bray J, Chennathukuzhi V, McNamara P, Curtis A, Zhang M, Blanchette-Mackie EJ, Strauss JF., IIIA sperm-associated WD repeat protein orthologous to Chlamydomonas PF20 associates with SPAG6, the mammalian orthologue of Chlamydomonas PF16. Mol Cell Biol 2002; 22: 7993–8004. - PMC - PubMed
-
- Zhang Z, Jones BH, Tang W, Moss SB, Wei Z, Ho C, Pollack M, Horowitz E, Bennet J, Baker ME, Strauss JF., IIIDissecting the axoneme interactome: the mammalian orthologue of Chlamydomonas PF6 interacts with sperm-associated antigen 6, the mammalian orthologue of Chlamydomonas PF16. Mol Cell Proteomics 2005; 4: 914–923. - PubMed
-
- Zhang Z, Kostetskii I, Tang W, Haig-Ladewig L, Sapiro R, Wei Z, Patel AM, Bennet J, Gerton GL, Moss SB, Radice GL, Strauss JF., IIIDeficiency of SPAG16L causes male infertility associated with impaired sperm motility. Biol Reprod 2006; 74: 751–759. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

