The annulus of the mouse sperm tail is required to establish a membrane diffusion barrier that is engaged during the late steps of spermiogenesis
- PMID: 20042538
- PMCID: PMC2842486
- DOI: 10.1095/biolreprod.109.079566
The annulus of the mouse sperm tail is required to establish a membrane diffusion barrier that is engaged during the late steps of spermiogenesis
Abstract
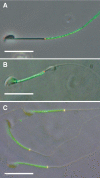
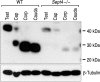
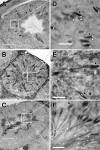
The annulus is a higher order septin cytoskeletal structure located between the midpiece and principal piece regions of the sperm tail. The annulus has been hypothesized to generate the diffusion barrier that exists between these two membrane domains. We tested this premise directly on septin 4 knockout mice, whose sperm are viable but lack an annulus, by following the diffusing membrane protein basigin. Basigin is normally confined to the principal piece domain on testicular and caput sperm, but undergoes relocation into the midpiece during sperm epididymal transit. On Sept4(-/-) sperm, domain confinement was lost, and basigin localized over the entire plasma membrane. Both immunofluorescence and immunoblotting further revealed reduced levels of basigin expression on sperm from the knockout. Testicular immunohistochemistry showed similar basigin expression and tail targeting in wild-type (WT) and Sept4(-/-) tubules until step 15 of spermatid development, at which point basigin was redistributed throughout the plasma membrane of Sept4(-/-) spermatids. The basigin outside of the tail was subsequently lost around the time of sperm release into the lumen. The redistribution in the knockout coincides with the time in WT sperm when the annulus completes its migration from the neck down to the midpiece-principal piece junction. We posit that basigin may not diffuse freely until after the annulus arrives at the midpiece-principal piece junction to restrict lateral movement. These results are the strongest evidence to date of a mammalian septin structure establishing a membrane diffusion barrier.
Figures





Similar articles
-
Spatiotemporal association of DNAJB13 with the annulus during mouse sperm flagellum development.BMC Dev Biol. 2009 Mar 19;9:23. doi: 10.1186/1471-213X-9-23. BMC Dev Biol. 2009. PMID: 19298648 Free PMC article.
-
Absence of annulus in human asthenozoospermia: case report.Hum Reprod. 2009 Jun;24(6):1296-303. doi: 10.1093/humrep/dep020. Epub 2009 Feb 15. Hum Reprod. 2009. PMID: 19221096
-
Septins at the annulus of mammalian sperm.Biol Chem. 2011 Aug;392(8-9):799-803. doi: 10.1515/BC.2011.074. Epub 2011 Jul 11. Biol Chem. 2011. PMID: 21740329 Review.
-
Cabs1 Maintains Structural Integrity of Mouse Sperm Flagella during Epididymal Transit of Sperm.Int J Mol Sci. 2021 Jan 11;22(2):652. doi: 10.3390/ijms22020652. Int J Mol Sci. 2021. PMID: 33440775 Free PMC article.
-
Different regulatory systems operate in the midpiece and principal piece of the mammalian sperm flagellum.Soc Reprod Fertil Suppl. 2007;65:331-4. Soc Reprod Fertil Suppl. 2007. PMID: 17644973 Review.
Cited by
-
Genetic mutation of Cep76 results in male infertility due to abnormal sperm tail composition.Life Sci Alliance. 2024 Apr 3;7(6):e202302452. doi: 10.26508/lsa.202302452. Print 2024 Jun. Life Sci Alliance. 2024. PMID: 38570187 Free PMC article.
-
Morphological and Molecular Bases of Male Infertility: A Closer Look at Sperm Flagellum.Genes (Basel). 2023 Feb 1;14(2):383. doi: 10.3390/genes14020383. Genes (Basel). 2023. PMID: 36833310 Free PMC article. Review.
-
Transition Zone Migration: A Mechanism for Cytoplasmic Ciliogenesis and Postaxonemal Centriole Elongation.Cold Spring Harb Perspect Biol. 2017 Aug 1;9(8):a028142. doi: 10.1101/cshperspect.a028142. Cold Spring Harb Perspect Biol. 2017. PMID: 28108487 Free PMC article. Review.
-
Septin filament formation is essential in budding yeast.Dev Cell. 2011 Apr 19;20(4):540-9. doi: 10.1016/j.devcel.2011.02.004. Dev Cell. 2011. PMID: 21497764 Free PMC article.
-
Localizations and functions of septins are susceptible to epitope tagging.bioRxiv [Preprint]. 2025 May 14:2025.05.13.653749. doi: 10.1101/2025.05.13.653749. bioRxiv. 2025. PMID: 40463252 Free PMC article. Preprint.
References
-
- Monesi V.Synthetic activities during spermatogenesis in the mouse. Exp Cell Res 1965; 39: 197–224. - PubMed
-
- Myles DG, Koppel DE, Cowan AE, Phelps BM, Primakoff P.Rearrangement of sperm surface antigens prior to fertilization. Ann N Y Acad Sci 1987; 513: 262–273. - PubMed
-
- Hunnicutt GR, Koppel DE, Myles DG.Analysis of the process of localization of fertilin to the sperm posterior head plasma membrane domain during sperm maturation in the epididymis. Dev Biol 1997; 191: 146–159. - PubMed
-
- Cowan AE, Koppel DE, Vargas LA, Hunnicutt GR.Guinea pig fertilin exhibits restricted lateral mobility in epididymal sperm and becomes freely diffusing during capacitation. Dev Biol 2001; 236: 502–509. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

