Matrix metalloproteinase-8 inactivates macrophage inflammatory protein-1 alpha to reduce acute lung inflammation and injury in mice
- PMID: 20042585
- PMCID: PMC2938777
- DOI: 10.4049/jimmunol.0900290
Matrix metalloproteinase-8 inactivates macrophage inflammatory protein-1 alpha to reduce acute lung inflammation and injury in mice
Abstract
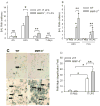
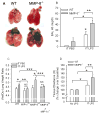
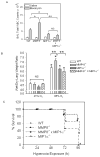
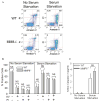
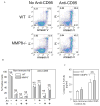
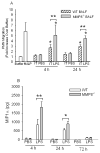
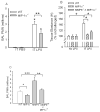
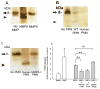
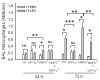
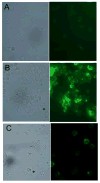
To determine the role of matrix metalloproteinase-8 (MMP-8) in acute lung injury (ALI), we delivered LPS or bleomycin by the intratracheal route to MMP-8(-/-) mice versus wild-type (WT) mice or subjected the mice to hyperoxia (95% O(2)) and measured lung inflammation and injury at intervals. MMP-8(-/-) mice with ALI had greater increases in lung polymorphonuclear neutrophils (PMNs) and macrophage counts, measures of alveolar capillary barrier injury, lung elastance, and mortality than WT mice with ALI. Bronchoalveolar lavage fluid (BALF) from LPS-treated MMP-8(-/-) mice had more MIP-1alpha than BALF from LPS-treated WT mice, but similar levels of other pro- and anti-inflammatory mediators. MIP-1alpha(-/-) mice with ALI had less acute lung inflammation and injury than WT mice with ALI, confirming that MIP-1alpha promotes acute lung inflammation and injury in mice. Genetically deleting MIP-1alpha in MMP-8(-/-) mice reduced the increased lung inflammation and injury and mortality in MMP-8(-/-) mice with ALI. Soluble MMP-8 cleaved and inactivated MIP-1alpha in vitro, but membrane-bound MMP-8 on activated PMNs had greater MIP-1alpha-degrading activity than soluble MMP-8. High levels of membrane-bound MMP-8 were detected on lung PMNs from LPS-treated WT mice, but soluble, active MMP-8 was not detected in BALF samples. Thus, MMP-8 has novel roles in restraining lung inflammation and in limiting alveolar capillary barrier injury during ALI in mice by inactivating MIP-1alpha. In addition, membrane-bound MMP-8 on activated lung PMNs is likely to be the key bioactive form of the enzyme that limits lung inflammation and alveolar capillary barrier injury during ALI.
Conflict of interest statement
None of the authors have conflicts of interest or financial interests to disclose.
Figures
Similar articles
-
Profibrotic activities for matrix metalloproteinase-8 during bleomycin-mediated lung injury.J Immunol. 2013 Apr 15;190(8):4283-96. doi: 10.4049/jimmunol.1201043. Epub 2013 Mar 13. J Immunol. 2013. PMID: 23487425 Free PMC article.
-
Absinthin attenuates LPS-induced ALI through MIP-1α-mediated inflammatory cell infiltration.Exp Lung Res. 2015;41(9):514-24. doi: 10.3109/01902148.2015.1093566. Exp Lung Res. 2015. PMID: 26495959
-
Regulation of inflammation by Rac2 in immune complex-mediated acute lung injury.Am J Physiol Lung Cell Mol Physiol. 2009 Dec;297(6):L1091-102. doi: 10.1152/ajplung.90471.2008. Epub 2009 Oct 2. Am J Physiol Lung Cell Mol Physiol. 2009. PMID: 19801448 Free PMC article.
-
Matrix metalloproteinase-8 deficiency promotes granulocytic allergen-induced airway inflammation.J Immunol. 2005 Aug 15;175(4):2589-97. doi: 10.4049/jimmunol.175.4.2589. J Immunol. 2005. PMID: 16081833
-
Protective effects of intratracheally administered quercetin on lipopolysaccharide-induced acute lung injury.Respir Res. 2014 Nov 21;15(1):150. doi: 10.1186/s12931-014-0150-x. Respir Res. 2014. PMID: 25413579 Free PMC article.
Cited by
-
Repair after acute lung injury: molecular mechanisms and therapeutic opportunities.Crit Care. 2012 Dec 12;16(2):209. doi: 10.1186/cc11224. Crit Care. 2012. PMID: 22429641 Free PMC article. Review. No abstract available.
-
Matrix metalloproteinase-9 deficiency protects mice from severe influenza A viral infection.JCI Insight. 2018 Dec 20;3(24):e99022. doi: 10.1172/jci.insight.99022. JCI Insight. 2018. PMID: 30568032 Free PMC article.
-
A Novel Role of Matrix Metalloproteinase-8 in Macrophage Differentiation and Polarization.J Biol Chem. 2015 Jul 31;290(31):19158-72. doi: 10.1074/jbc.M114.634022. Epub 2015 Jun 19. J Biol Chem. 2015. PMID: 26092731 Free PMC article.
-
Machine Learning Identifies Complicated Sepsis Course and Subsequent Mortality Based on 20 Genes in Peripheral Blood Immune Cells at 24 H Post-ICU Admission.Front Immunol. 2021 Feb 22;12:592303. doi: 10.3389/fimmu.2021.592303. eCollection 2021. Front Immunol. 2021. PMID: 33692779 Free PMC article.
-
Contribution of neutrophils to acute lung injury.Mol Med. 2011 Mar-Apr;17(3-4):293-307. doi: 10.2119/molmed.2010.00138. Epub 2010 Oct 18. Mol Med. 2011. PMID: 21046059 Free PMC article. Review.
References
-
- Owen CA, Campbell EJ. The cell biology of leukocyte-mediated proteolysis. J Leukoc Biol. 1999;65:137–150. - PubMed
-
- Owen CA, Hu Z, Lopez-Otin C, Shapiro SD. Membrane-bound matrix metalloproteinase-8 on activated polymorphonuclear cells is a potent, tissue inhibitor of metalloproteinase-resistant collagenase and serpinase. J Immunol. 2004;172:7791–7803. - PubMed
-
- Balbin M, Fueyo A, Knauper V, Pendas AM, Lopez JM, Jimenez MG, Murphy G, Lopez-Otin C. Collagenase 2 (MMP-8) expression in murine tissue-remodeling processes. Analysis of its potential role in postpartum involution of the uterus. J Biol Chem. 1998;273:23959–23968. - PubMed
-
- Balbin M, Fueyo A, Tester AM, Pendas AM, Pitiot AS, Astudillo A, Overall CM, Shapiro SD, Lopez-Otin C. Loss of collagenase-2 confers increased skin tumor susceptibility to male mice. Nat Genet. 2003;35:252–257. - PubMed
-
- Cowland JB, Borregaard N. The individual regulation of granule protein mRNA levels during neutrophil maturation explains the heterogeneity of neutrophil granules. J Leukoc Biol. 1999;66:989–995. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

