Critical role of IL-1 receptor-associated kinase-M in regulating chemokine-dependent deleterious inflammation in murine influenza pneumonia
- PMID: 20042589
- PMCID: PMC2995366
- DOI: 10.4049/jimmunol.0901709
Critical role of IL-1 receptor-associated kinase-M in regulating chemokine-dependent deleterious inflammation in murine influenza pneumonia
Abstract
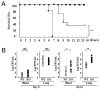
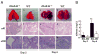
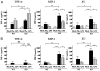
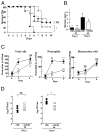
Influenza virus is a common cause of respiratory infection and morbidity, which is often due to deleterious host immune responses directed against the pathogen. We investigated the role of IL-1 receptor-associated kinase-M (IRAK-M), an inhibitor of MyD88-dependent TLR signaling, in modulating the innate inflammatory response during influenza pneumonia using a murine model. The intranasal administration of influenza resulted in the upregulation of IRAK-M mRNA and protein levels in the lungs within 2 d after infectious challenge. Pulmonary influenza infection in mice deficient in IRAK-M (IRAK-M(-/-)) resulted in substantially increased mortality compared with similarly treated wild-type animals. Increased mortality in IRAK-M(-/-) mice was associated with enhanced early influx of neutrophils, high permeability edema, apoptosis of lung epithelial cells, markedly increased expression of inflammatory cytokines/chemokines, and release of neutrophil-derived enzymes, including myeloperoxidase and neutrophil elastase. Early viral clearance was not different in mutant mice, whereas viral titers in lungs and blood were significantly higher in IRAK-M(-/-) mice compared with wild-type animals. Increased lethality observed in IRAK-M(-/-) mice after influenza challenge was abrogated by Ab-mediated blockade of CXCR2. Collectively, our findings indicate that IRAK-M is critical to preventing deleterious neutrophil-dependent lung injury during influenza infection of the respiratory tract.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures




Similar articles
-
TLR signaling prevents hyperoxia-induced lung injury by protecting the alveolar epithelium from oxidant-mediated death.J Immunol. 2012 Jul 1;189(1):356-64. doi: 10.4049/jimmunol.1103124. Epub 2012 Jun 1. J Immunol. 2012. PMID: 22661086 Free PMC article.
-
IL-36 receptor deletion attenuates lung injury and decreases mortality in murine influenza pneumonia.Mucosal Immunol. 2017 Jul;10(4):1043-1055. doi: 10.1038/mi.2016.107. Epub 2016 Dec 14. Mucosal Immunol. 2017. PMID: 27966554 Free PMC article.
-
Interleukin-1 receptor-associated kinase M-deficient mice demonstrate an improved host defense during Gram-negative pneumonia.Mol Med. 2012 Sep 25;18(1):1067-75. doi: 10.2119/molmed.2011.00450. Mol Med. 2012. PMID: 22729155 Free PMC article.
-
Dysregulated macrophage-inflammatory protein-2 expression drives illness in bacterial superinfection of influenza.J Immunol. 2010 Feb 15;184(4):2001-13. doi: 10.4049/jimmunol.0903304. Epub 2010 Jan 11. J Immunol. 2010. PMID: 20065113
-
The interleukin-1 receptor-associated kinases: critical regulators of innate immune signalling.Biochem Pharmacol. 2010 Dec 15;80(12):1981-91. doi: 10.1016/j.bcp.2010.06.020. Epub 2010 Jun 23. Biochem Pharmacol. 2010. PMID: 20599782 Review.
Cited by
-
Macrophage phenotype controls long-term AKI outcomes--kidney regeneration versus atrophy.J Am Soc Nephrol. 2014 Feb;25(2):292-304. doi: 10.1681/ASN.2013020152. Epub 2013 Dec 5. J Am Soc Nephrol. 2014. PMID: 24309188 Free PMC article.
-
NMP4 regulates the innate immune response to influenza A virus infection.Mucosal Immunol. 2021 Jan;14(1):209-218. doi: 10.1038/s41385-020-0280-z. Epub 2020 Mar 9. Mucosal Immunol. 2021. PMID: 32152414 Free PMC article.
-
TLR signaling prevents hyperoxia-induced lung injury by protecting the alveolar epithelium from oxidant-mediated death.J Immunol. 2012 Jul 1;189(1):356-64. doi: 10.4049/jimmunol.1103124. Epub 2012 Jun 1. J Immunol. 2012. PMID: 22661086 Free PMC article.
-
Gender Difference in Perceived Symptoms and Laboratory Investigations in Suspected and Confirmed COVID-19 Cases: A Retrospective Study.J Prim Care Community Health. 2021 Jan-Dec;12:21501327211039718. doi: 10.1177/21501327211039718. J Prim Care Community Health. 2021. PMID: 34407661 Free PMC article.
-
IL-36 receptor deletion attenuates lung injury and decreases mortality in murine influenza pneumonia.Mucosal Immunol. 2017 Jul;10(4):1043-1055. doi: 10.1038/mi.2016.107. Epub 2016 Dec 14. Mucosal Immunol. 2017. PMID: 27966554 Free PMC article.
References
-
- Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM, Jr, Musher DM, Niederman MS, et al. Infectious Diseases Society of America; American Thoracic Society. 2007. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 44(Suppl 2):S27–S72. - PMC - PubMed
-
- Glezen WP, Greenberg SB, Atmar RL, Piedra PA, Couch RB. Impact of respiratory virus infections on persons with chronic underlying conditions. JAMA. 2000;283:499–505. - PubMed
-
- Murphy BR, Webster R. Fields Virology. 3. Lippencott-Raven; Philadelphia: 1996. pp. 1397–1445.
-
- Woodhead M, Blasi F, Ewig S, Huchon G, Ieven M, Leven M, Ortqvist A, Schaberg T, Torres A, van der Heijden G, Verheij TJ European Respiratory Society; European Society of Clinical Microbiology and Infectious Diseases. Guidelines for the management of adult lower respiratory tract infections. Eur Respir J. 2005;26:1138–1180. - PubMed
-
- Zuckerman AJ, Banatvala J, Pattison JR, Griffiths PD, Schoub BD. Principals and Practice of Clnical Virology. 5. John Wiley and Sons; West Sussex, England: 2004.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

