Nickel ions inhibit histone demethylase JMJD1A and DNA repair enzyme ABH2 by replacing the ferrous iron in the catalytic centers
- PMID: 20042601
- PMCID: PMC2844186
- DOI: 10.1074/jbc.M109.058503
Nickel ions inhibit histone demethylase JMJD1A and DNA repair enzyme ABH2 by replacing the ferrous iron in the catalytic centers
Erratum in
-
Nickel ions inhibit histone demethylase JMJD1A and DNA repair enzyme ABH2 by replacing the ferrous iron in the catalytic centers.J Biol Chem. 2017 Jun 23;292(25):10743. doi: 10.1074/jbc.A109.058503. J Biol Chem. 2017. PMID: 28646124 Free PMC article. No abstract available.
Abstract
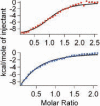
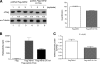
Iron- and 2-oxoglutarate-dependent dioxygenases are a diverse family of non-heme iron enzymes that catalyze various important oxidations in cells. A key structural motif of these dioxygenases is a facial triad of 2-histidines-1-carboxylate that coordinates the Fe(II) at the catalytic site. Using histone demethylase JMJD1A and DNA repair enzyme ABH2 as examples, we show that this family of dioxygenases is highly sensitive to inhibition by carcinogenic nickel ions. We find that, with iron, the 50% inhibitory concentrations of nickel (IC(50) [Ni(II)]) are 25 microm for JMJD1A and 7.5 microm for ABH2. Without iron, JMJD1A is 10 times more sensitive to nickel inhibition with an IC(50) [Ni(II)] of 2.5 microm, and approximately one molecule of Ni(II) inhibits one molecule of JMJD1A, suggesting that nickel causes inhibition by replacing the iron. Furthermore, nickel-bound JMJD1A is not reactivated by excessive iron even up to a 2 mm concentration. Using x-ray absorption spectroscopy, we demonstrate that nickel binds to the same site in ABH2 as iron, and replacement of the iron by nickel does not prevent the binding of the cofactor 2-oxoglutarate. Finally, we show that nickel ions target and inhibit JMJD1A in intact cells, and disruption of the iron-binding site decreases binding of nickel ions to ABH2 in intact cells. Together, our results reveal that the members of this dioxygenase family are specific targets for nickel ions in cells. Inhibition of these dioxygenases by nickel is likely to have widespread impacts on cells (e.g. impaired epigenetic programs and DNA repair) and may eventually lead to cancer development.
Figures





References
-
- Grandjean P., Andersen O., Nielsen G. D. (1988) Am. J. Ind. Med. 13, 193–209 - PubMed
-
- Costa M., Simmons-Hansen J., Bedrossian C. W., Bonura J., Caprioli R. M. (1981) Cancer Res. 41, 2868–2876 - PubMed
-
- Ke Q., Davidson T., Kluz T., Oller A., Costa M. (2007) Toxicol. Appl. Pharmacol. 219, 18–23 - PubMed
-
- Costa M., Abbracchio M. P., Simmons-Hansen J. (1981) Toxicol. Appl. Pharmacol. 60, 313–323 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

