Evidence for proteotoxicity in beta cells in type 2 diabetes: toxic islet amyloid polypeptide oligomers form intracellularly in the secretory pathway
- PMID: 20042670
- PMCID: PMC2808091
- DOI: 10.2353/ajpath.2010.090532
Evidence for proteotoxicity in beta cells in type 2 diabetes: toxic islet amyloid polypeptide oligomers form intracellularly in the secretory pathway
Abstract
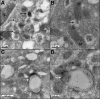
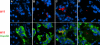
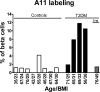
The islet in type 2 diabetes mellitus (T2DM) is characterized by a deficit in beta cells and islet amyloid derived from islet amyloid polypeptide (IAPP), a protein co-expressed with insulin by beta cells. It is increasingly appreciated that the toxic form of amyloidogenic proteins is not amyloid but smaller membrane-permeant oligomers. Using an antibody specific for toxic oligomers and cryo-immunogold labeling in human IAPP transgenic mice, human insulinoma and pancreas from humans with and without T2DM, we sought to establish the abundance and sites of formation of IAPP toxic oligomers. We conclude that IAPP toxic oligomers are formed intracellularly within the secretory pathway in T2DM. Most striking, IAPP toxic oligomers appear to disrupt membranes of the secretory pathway, and then when adjacent to mitochondria, disrupt mitochondrial membranes. Toxic oligomer-induced secretory pathway and mitochondrial membrane disruption is a novel mechanism to account for cellular dysfunction and apoptosis in T2DM.
Figures




References
-
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. - PubMed
-
- Ohsawa H, Kanatsuka A, Yamaguchi T, Makino H, Yoshida S. Islet amyloid polypeptide inhibits glucose-stimulated insulin secretion from isolated rat pancreatic islets. Biochem Biophys Res Commun. 1989;160:961–967. - PubMed
-
- Butler PC, Chou J, Carter WB, Wang YN, Bu BH, Chang D, Chang JK, Rizza RA. Effects of meal ingestion on plasma amylin concentration in NIDDM and nondiabetic humans. Diabetes. 1990;39:752–756. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

