Lung chitinolytic activity and chitotriosidase are elevated in chronic obstructive pulmonary disease and contribute to lung inflammation
- PMID: 20042671
- PMCID: PMC2808072
- DOI: 10.2353/ajpath.2010.090455
Lung chitinolytic activity and chitotriosidase are elevated in chronic obstructive pulmonary disease and contribute to lung inflammation
Abstract
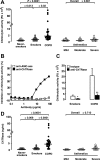
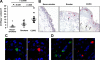
Chronic obstructive pulmonary disease (COPD) is characterized by chronic airway inflammation and emphysematous alveolar destruction. In this study, we have investigated whether chitotriosidase (ChTRase) and acidic mammalian chitinase, two chitinases with chitinolytic activity, are selectively augmented in COPD and contribute to its pathogenesis. We found that smokers with COPD, but not asthmatics, had higher chitinolytic activity and increased levels of ChTRase in bronchoalveolar lavage, more ChTRase-positive cells in bronchial biopsies, and an elevated proportion of alveolar macrophages expressing ChTRase than smokers without COPD or never-smokers. ChTRase accounted for approximately 80% of bronchoalveolar lavage chitinolytic activity, while acidic mammalian chitinase was undetectable. Bronchoalveolar lavage chitinolytic activity and ChTRase were associated with airflow obstruction and emphysema and with the levels of interleukin (IL)-1beta, IL-8, tumor-necrosis factor (TNF)-alpha, and its type II soluble receptor. Tumor necrosis factor-alpha stimulated ChTRase release only from alveolar macrophages from smokers with COPD, and exposure of these cells to ChTRase promoted the release of IL-8, monocyte-chemoattractant protein-1, and metalloproteinase-9. Finally, ChTRase overexpression in the lung of normal mice promoted macrophage recruitment and the synthesis of the murine homologue of IL-8, keratinocyte-derived cytokine, and of monocyte-chemoattractant protein-1. We conclude that pulmonary ChTRase overexpression may represent a novel important mechanism involved in COPD onset and progression.
Figures


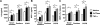
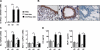
Similar articles
-
YKL-40 is elevated in patients with chronic obstructive pulmonary disease and activates alveolar macrophages.J Immunol. 2008 Oct 1;181(7):5167-73. doi: 10.4049/jimmunol.181.7.5167. J Immunol. 2008. PMID: 18802121
-
Chitotriosidase is the primary active chitinase in the human lung and is modulated by genotype and smoking habit.J Allergy Clin Immunol. 2008 Nov;122(5):944-950.e3. doi: 10.1016/j.jaci.2008.08.023. Epub 2008 Oct 9. J Allergy Clin Immunol. 2008. PMID: 18845328 Free PMC article.
-
The activity and expression of chitinase in the equine lung and its activity in normal horses and animals with recurrent airway obstruction.Res Vet Sci. 2009 Aug;87(1):20-5. doi: 10.1016/j.rvsc.2008.11.002. Epub 2008 Dec 21. Res Vet Sci. 2009. PMID: 19103451
-
Inflammatory cells and chronic obstructive pulmonary disease.Curr Drug Targets Inflamm Allergy. 2005 Dec;4(6):607-18. doi: 10.2174/156801005774912824. Curr Drug Targets Inflamm Allergy. 2005. PMID: 17305517 Review.
-
Macrophage Polarization and Functions in Pathogenesis of Chronic Obstructive Pulmonary Disease.Int J Mol Sci. 2024 May 22;25(11):5631. doi: 10.3390/ijms25115631. Int J Mol Sci. 2024. PMID: 38891820 Free PMC article. Review.
Cited by
-
Macrophage heterogeneity in respiratory diseases.Mediators Inflamm. 2013;2013:769214. doi: 10.1155/2013/769214. Epub 2013 Feb 27. Mediators Inflamm. 2013. PMID: 23533311 Free PMC article. Review.
-
A Plumieridine-Rich Fraction From Allamanda polyantha Inhibits Chitinolytic Activity and Exhibits Antifungal Properties Against Cryptococcus neoformans.Front Microbiol. 2020 Aug 28;11:2058. doi: 10.3389/fmicb.2020.02058. eCollection 2020. Front Microbiol. 2020. PMID: 32983042 Free PMC article.
-
Irreversible evolutionary loss of chitin-degrading ability in the chitinase-like protein Ym1 under positive selection in rodents.Protein Sci. 2023 Apr;32(4):e4620. doi: 10.1002/pro.4620. Protein Sci. 2023. PMID: 36883357 Free PMC article.
-
Heterogeneity of lung mononuclear phagocytes in chronic obstructive pulmonary disease.J Innate Immun. 2012;4(5-6):489-97. doi: 10.1159/000337434. Epub 2012 May 3. J Innate Immun. 2012. PMID: 22572241 Free PMC article. Review.
-
Exploratory Longitudinal Analysis of the Circulating CHIT1 Activity in Pediatric Patients with Obesity.Children (Basel). 2023 Jan 6;10(1):124. doi: 10.3390/children10010124. Children (Basel). 2023. PMID: 36670674 Free PMC article.
References
-
- Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med. 2000;343:269–280. - PubMed
-
- Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet. 2004;364:709–721. - PubMed
-
- Barnes PJ. Mediators of chronic obstructive pulmonary disease. Pharmacol Rev. 2004;56:515–548. - PubMed
-
- Barnes PJ. Alveolar macrophages as orchestrators of COPD. COPD. 2004;1:59–70. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

