Expression of the embryonic stem cell transcription factor SOX2 in human skin: relevance to melanocyte and merkel cell biology
- PMID: 20042675
- PMCID: PMC2808095
- DOI: 10.2353/ajpath.2010.090495
Expression of the embryonic stem cell transcription factor SOX2 in human skin: relevance to melanocyte and merkel cell biology
Abstract
SOX2 is a gene located on chromosome 3q26.33 that encodes a transcription factor important to maintenance of embryonic neural crest stem cell pluripotency. We have identified rare SOX2-immunoreactive cells in normal human skin at or near the established stem cell niches. Three subsets of SOX2-positive cells were defined in these regions: those expressing only SOX2 and those that co-expressed SOX2 and either CK20 or microphthalmia-associated transcription factor, which are consistent with dichotomous differentiation of SOX2-expressing precursors along neuroendocrine (Merkel cell) or melanocytic lines, respectively. Examination of Merkel cell carcinomas confirmed nuclear SOX2 expression in this tumor type. In human patient melanoma, strong nuclear expression of SOX2 was noted in a subset of tumors, and the ability to detect SOX2 in lesional cells significantly correlated with primary tumor thickness in a survey cohort. To assess the potential role of SOX2 in melanoma growth, an in vivo tumorigenesis assay was used. Whereas SOX2 knockdown failed to influence proliferation of cultured melanoma cells in vitro, tumor xenografts generated with the SOX2-knockdown cell line showed significant decrease in mean tumor volume as compared with controls. In aggregate, these findings suggest that SOX2 is a novel biomarker for subpopulations of normal skin cells that reside in established stem cell niches and that might relate to Merkel cell and melanocyte ontogeny and tumorigenesis.
Figures
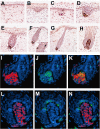
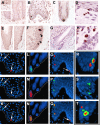



References
-
- Bittner M, Meltzer P, Chen Y, Jiang Y, Seftor E, Hendrix M, Radmacher M, Simon Rm, Yakhini Z, Ben-Dor A, Sampas N, Dougherty E, Wang E, Marincola F, Gooden C, Lueders J, Glatfelter A, Pollock P, Carpten J, Gillanders E, Leja D, Dietrich K, Beaudry C, Berens M, Alberts D, Sondak V. Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature. 2000;406:536–540. - PubMed
-
- Hendrix MJ, Seftor EA, Hess AR, Seftor RE. Molecular plasticity of human melanoma cells. Oncogene. 2003;22:3070–3075. - PubMed
-
- Simpson AJ, Caballero OL, Jungbulth A, Chen YT, Old LJ. Cancer/testis antigens, gametogenesis, and cancer. Nat Rev Cancer. 2005;5:615–625. - PubMed
-
- Rothhammer T, Wild PJ, Meyer S, Bataille F, Pauer F, Pauer A, Klinkhammer-Schalke M, Hein R, Hofstaedter F, Bosserhoff AK. Bone morphogenic protein 7 (BMP7) expression is a potential novel prognostic marker for recurrence in patients with primary melanoma. Cancer Biomark. 2007;3:111–117. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

