Memantine improves cognition and reduces Alzheimer's-like neuropathology in transgenic mice
- PMID: 20042680
- PMCID: PMC2808092
- DOI: 10.2353/ajpath.2010.090452
Memantine improves cognition and reduces Alzheimer's-like neuropathology in transgenic mice
Abstract
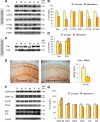
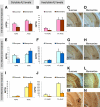
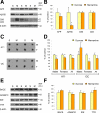
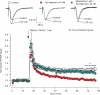
Memantine is an N-methyl-d-aspartate receptor antagonist that is approved for the treatment of moderate to severe Alzheimer's disease (AD). In this study, three groups of triple-transgenic (3xTg-AD) mice with differing levels of AD-like pathology (6, 9, and 15 months of age) were treated for 3 months with doses of memantine equivalent to those used in humans. After the treatment, memantine-treated mice had restored cognition and significantly reduced the levels of insoluble amyloid-beta (Abeta), Abeta dodecamers (Abeta*56), prefibrillar soluble oligomers, and fibrillar oligomers. The effects on pathology were stronger in older, more impaired animals. Memantine treatment also was associated with a decline in the levels of total tau and hyperphosphorylated tau. Finally, memantine pre-incubation prevented Abeta-induced inhibition of long-term potentiation in hippocampal slices of cognitively normal mice. These results suggest that the effects of memantine treatment on AD brain include disease modification and prevention of synaptic dysfunction.
Figures

Comment in
-
Memantine: "hypothesis testing" not "disease modifying" in Alzheimer's disease.Am J Pathol. 2010 Feb;176(2):540-1. doi: 10.2353/ajpath.2010.090856. Epub 2009 Dec 17. Am J Pathol. 2010. PMID: 20019185 Free PMC article.
Similar articles
-
Peripheral and Central Effects of Memantine in a Mixed Preclinical Mice Model of Obesity and Familial Alzheimer's Disease.Mol Neurobiol. 2018 Sep;55(9):7327-7339. doi: 10.1007/s12035-018-0868-4. Epub 2018 Feb 5. Mol Neurobiol. 2018. PMID: 29404958
-
Ebselen ameliorates β-amyloid pathology, tau pathology, and cognitive impairment in triple-transgenic Alzheimer's disease mice.J Biol Inorg Chem. 2017 Aug;22(6):851-865. doi: 10.1007/s00775-017-1463-2. Epub 2017 May 13. J Biol Inorg Chem. 2017. PMID: 28502066
-
Memantine protects rat cortical cultured neurons against beta-amyloid-induced toxicity by attenuating tau phosphorylation.Eur J Neurosci. 2008 Nov;28(10):1989-2002. doi: 10.1111/j.1460-9568.2008.06498.x. Eur J Neurosci. 2008. PMID: 19046381
-
[Memantine: from the original brand to generics].Zh Nevrol Psikhiatr Im S S Korsakova. 2017;117(10):136-143. doi: 10.17116/jnevro2017117101136-143. Zh Nevrol Psikhiatr Im S S Korsakova. 2017. PMID: 29171502 Review. Russian.
-
Effects of CX3CR1 and Fractalkine Chemokines in Amyloid Beta Clearance and p-Tau Accumulation in Alzheimer's Disease (AD) Rodent Models: Is Fractalkine a Systemic Biomarker for AD?Curr Alzheimer Res. 2016;13(4):403-12. doi: 10.2174/1567205013666151116125714. Curr Alzheimer Res. 2016. PMID: 26567742 Review.
Cited by
-
Effects of Dizocilpine, Midazolam and Their Co-Application on the Trimethyltin (TMT)-Induced Rat Model of Cognitive Deficit.Brain Sci. 2021 Mar 22;11(3):400. doi: 10.3390/brainsci11030400. Brain Sci. 2021. PMID: 33809889 Free PMC article.
-
Amyloid Beta-Related Alterations to Glutamate Signaling Dynamics During Alzheimer's Disease Progression.ASN Neuro. 2019 Jan-Dec;11:1759091419855541. doi: 10.1177/1759091419855541. ASN Neuro. 2019. PMID: 31213067 Free PMC article. Review.
-
Memantine add on to citalopram in elderly patients with depression: A double-blind placebo-controlled study.J Res Med Sci. 2014 Jun;19(6):525-30. J Res Med Sci. 2014. PMID: 25197294 Free PMC article.
-
Cognitive Decline in Preclinical Alzheimer's Disease: Amyloid-Beta versus Tauopathy.J Alzheimers Dis. 2018;61(1):265-281. doi: 10.3233/JAD-170490. J Alzheimers Dis. 2018. PMID: 29154274 Free PMC article. Review.
-
Long-term pioglitazone treatment improves learning and attenuates pathological markers in a mouse model of Alzheimer's disease.J Alzheimers Dis. 2012;30(4):943-61. doi: 10.3233/JAD-2012-111661. J Alzheimers Dis. 2012. PMID: 22495349 Free PMC article.
References
-
- Alzheimer’s Association Chicago: Alzheimer’s Association,; Alzheimer’s Disease Facts and Figures 2007. 2007
-
- Hui JS, Wilson RS, Bennett DA, Bienias JL, Gilley DW, Evans DA. Rate of cognitive decline and mortality in Alzheimer’s disease. Neurology. 2003;61:1356–1361. - PubMed
-
- Honig LS, Mayeux R. Natural history of Alzheimer’s disease. Aging (Milano) 2001;13:171–182. - PubMed
-
- Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. - PubMed
-
- Selkoe DJ. Alzheimer’s disease results from the cerebral accumulation and cytotoxicity of amyloid beta-protein. J Alzheimers Dis. 2001;3:75–80. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

