A SLC4-like anion exchanger from renal tubules of the mosquito (Aedes aegypti): evidence for a novel role of stellate cells in diuretic fluid secretion
- PMID: 20042685
- PMCID: PMC2838654
- DOI: 10.1152/ajpregu.00729.2009
A SLC4-like anion exchanger from renal tubules of the mosquito (Aedes aegypti): evidence for a novel role of stellate cells in diuretic fluid secretion
Abstract
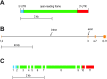
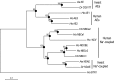
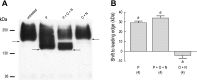
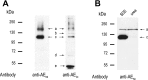
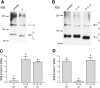
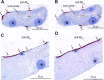
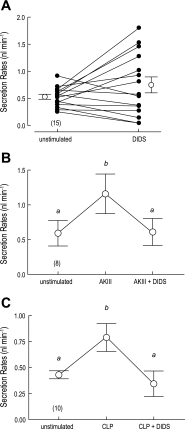
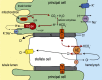
Transepithelial fluid secretion across the renal (Malpighian) tubule epithelium of the mosquito (Aedes aegypti) is energized by the vacuolar-type (V-type) H(+)-ATPase and not the Na(+)-K(+)-ATPase. Located at the apical membrane of principal cells, the V-type H(+)-ATPase translocates protons from the cytoplasm to the tubule lumen. Secreted protons are likely to derive from metabolic H(2)CO(3), which raises questions about the handling of HCO(3)(-) by principal cells. Accordingly, we tested the hypothesis that a Cl/HCO(3) anion exchanger (AE) related to the solute-linked carrier 4 (SLC4) superfamily mediates the extrusion of HCO(3)(-) across the basal membrane of principal cells. We began by cloning from Aedes Malpighian tubules a full-length cDNA encoding an SLC4-like AE, termed AeAE. When expressed heterologously in Xenopus oocytes, AeAE is both N- and O-glycosylated and mediates Na(+)-independent intracellular pH changes that are sensitive to extracellular Cl(-) concentration and to DIDS. In Aedes Malpighian tubules, AeAE is expressed as two distinct forms: one is O-glycosylated, and the other is N-glycosylated. Significantly, AeAE immunoreactivity localizes to the basal regions of stellate cells but not principal cells. Concentrations of DIDS that inhibit AeAE activity in Xenopus oocytes have no effects on the unstimulated rates of fluid secretion mediated by Malpighian tubules as measured by the Ramsay assay. However, in Malpighian tubules stimulated with kinin or calcitonin-like diuretic peptides, DIDS reduces the diuretic rates of fluid secretion to basal levels. In conclusion, Aedes Malpighian tubules express AeAE in the basal region of stellate cells, where this transporter may participate in producing diuretic rates of transepithelial fluid secretion.
Figures





References
-
- Beyenbach KW. Energizing epithelial transport with the vacuolar H+-ATPase. News Physiol Sci 16: 145–151, 2001. - PubMed
-
- Beyenbach KW. Regulation of tight junction permeability with switch-like speed. Curr Opin Nephrol Hypertens 12: 543–550, 2003. - PubMed
-
- Beyenbach KW. Transport mechanisms of diuresis in Malpighian tubules of insects. J Exp Biol 206: 3845–3856, 2003. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

