Follicle-stimulating hormone, interleukin-1, and bone density in adult women
- PMID: 20042686
- PMCID: PMC2838653
- DOI: 10.1152/ajpregu.00728.2009
Follicle-stimulating hormone, interleukin-1, and bone density in adult women
Abstract
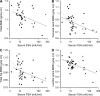
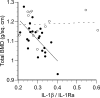
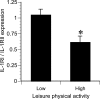
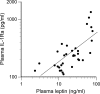
Recent studies have indicated that follicle-stimulating hormone (FSH) promotes bone loss. The present study tested the hypothesis that FSH enhances the activity of bone-resorbing cytokines [interleukin (IL)-1beta, tumor necrosis factor (TNF)-alpha, and IL-6], either by inducing their secretion or by altering their receptor expression. Thirty-six women between the ages of 20 and 50 were assessed for bone mineral density (BMD), reproductive hormone, cytokine ligand and soluble receptor concentrations, and surface expression of cytokine receptors on monocytes. In addition, isolated mononuclear cells were incubated in vitro with exogenous FSH. Univariate regression analyses indicated that BMD was inversely related to serum FSH (r = -0.29 to -0.51, P = 0.03-0.001, depending upon the skeletal site). Physical activity and body composition were also identified as significant factors by multiple regressions. Exogenous FSH induced isolated cells to secrete IL-1beta, TNF-alpha, and IL-6 in proportion to the surface expression of FSH receptors on the monocytes. Endogenous (serum) FSH concentrations correlated with the circulating concentrations of these cytokines. None of these individual cytokines was related to BMD, but the IL-1beta to IL-1 receptor antagonist (IL-1Ra) ratio was inversely related to BMD (r = -0.53, P = 0.002) in all but the most physically active women, who had significantly lower expression of IL-1 type I receptors relative to type II (decoy receptors, P = 0.01). Physical activity also correlated positively with secretion of inhibitory soluble IL-1 receptors (r = 0.53, P = 0.003). Moreover, IL-1Ra correlated strongly with percent body fat (r = 0.66, P < 0.0001). These results indicate that BMD is related to FSH concentration, physical activity, and body composition. Although each of these factors likely has direct effects on bone, the present study suggests that each may also influence BMD by modulating the activity of the osteoresorptive cytokine IL-1beta.
Figures

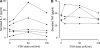

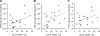


References
-
- Abrahamsen B, Bonnevie-Nielsen V, Ebbesen EN, Gram J, Beck-Nielsen H. Cytokines and bone loss in a 5-year longitudinal study Hormone replacement therapy suppresses serum soluble interleukin-6 receptor and increases interleukin-1 receptor antagonist: The Danish Osteoporosis Prevention Study. J Bone Miner Res 15: 1545–1554, 2000 - PubMed
-
- Arend WP. The mode of action of cytokine inhibitors. J Rheumatol 29, Suppl 65: 16–21, 2002 - PubMed
-
- Athreya BH, Pletcher J, Zulian F, Weiner DB, Williams WV. Subset-specific effects of sex hormones and pituitary gonadotropins on human lymphocyte proliferation in vitro. Clin Immunol Immunopathol 66: 201–211, 1993 - PubMed
-
- Atkins GJ, Kostakis P, Vincent C, Farrugia AN, Houchins JP, Findley DM, Evdokiou A, Zannettino ACW. RANK expression as a cell surface marker of human osteoclast precursors in peripheral blood, bone marrow, and giant cell tumors of bone. J Bone Miner Res 21: 1339–1349, 2006 - PubMed
-
- Blain H, Vuillemin A, Guillemin F, Durant R, Hanesse B, de Talance N, Doucet B, Jeandel C. Serum leptin level is a predictor of bone mineral density in postmenopausal women. J Clin Endo Metab 87: 1030–1035, 2002 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

