Long-lasting synaptic potentiation induced by depolarization under conditions that eliminate detectable Ca2+ signals
- PMID: 20042699
- PMCID: PMC2887625
- DOI: 10.1152/jn.00704.2009
Long-lasting synaptic potentiation induced by depolarization under conditions that eliminate detectable Ca2+ signals
Abstract
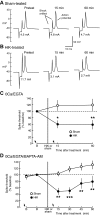
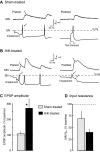
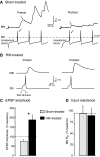
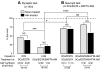
Activity-dependent alterations of synaptic transmission important for learning and memory are often induced by Ca(2+) signals generated by depolarization. While it is widely assumed that Ca(2+) is the essential transducer of depolarization into cellular plasticity, little effort has been made to test whether Ca(2+)-independent responses to depolarization might also induce memory-like alterations. It was recently discovered that peripheral axons of nociceptive sensory neurons in Aplysia display long-lasting hyperexcitability triggered by conditioning depolarization in the absence of Ca(2+) entry (using nominally Ca(2+)-free solutions containing EGTA, "0Ca/EGTA") or the absence of detectable Ca(2+) transients (adding BAPTA-AM, "0Ca/EGTA/BAPTA-AM"). The current study reports that depolarization of central ganglia to approximately 0 mV for 2 min in these same solutions induced hyperexcitability lasting >1 h in sensory neuron processes near their synapses onto motor neurons. Furthermore, conditioning depolarization in these solutions produced a 2.5-fold increase in excitatory postsynaptic potential (EPSP) amplitude 1-3 h afterward despite a drop in motor neuron input resistance. Depolarization in 0 Ca/EGTA produced long-term potentiation (LTP) of the EPSP lasting > or = 1 days without changing postsynaptic input resistance. When re-exposed to extracellular Ca(2+) during synaptic tests, prior exposure to 0Ca/EGTA or to 0Ca/EGTA/BAPTA-AM decreased sensory neuron survival. However, differential effects on neuronal health are unlikely to explain the observed potentiation because conditioning depolarization in these solutions did not alter survival rates. These findings suggest that unrecognized Ca(2+)-independent signals can transduce depolarization into long-lasting synaptic potentiation, perhaps contributing to persistent synaptic alterations following large, sustained depolarizations that occur during learning, neural injury, or seizures.
Figures


Similar articles
-
Long-lasting hyperexcitability induced by depolarization in the absence of detectable Ca2+ signals.J Neurophysiol. 2009 Mar;101(3):1351-60. doi: 10.1152/jn.91012.2008. Epub 2009 Jan 14. J Neurophysiol. 2009. PMID: 19144743 Free PMC article.
-
Contribution of postsynaptic Ca2+ to the induction of post-tetanic potentiation in the neural circuit for siphon withdrawal in Aplysia.J Neurosci. 2001 Mar 1;21(5):1739-49. doi: 10.1523/JNEUROSCI.21-05-01739.2001. J Neurosci. 2001. PMID: 11222663 Free PMC article.
-
Taurine-induced synaptic potentiation: role of calcium and interaction with LTP.Neuropharmacology. 2000;39(1):40-54. doi: 10.1016/s0028-3908(99)00078-7. Neuropharmacology. 2000. PMID: 10665818
-
Postsynaptic regulation of the development and long-term plasticity of Aplysia sensorimotor synapses in cell culture.J Neurobiol. 1994 Jun;25(6):666-93. doi: 10.1002/neu.480250608. J Neurobiol. 1994. PMID: 8071666 Review.
-
Hippocampal long-term synaptic plasticity and signal amplification of NMDA receptors.Crit Rev Neurobiol. 2006;18(1-2):71-84. doi: 10.1615/critrevneurobiol.v18.i1-2.80. Crit Rev Neurobiol. 2006. PMID: 17725510 Review.
Cited by
-
Nociceptive Biology of Molluscs and Arthropods: Evolutionary Clues About Functions and Mechanisms Potentially Related to Pain.Front Physiol. 2018 Aug 3;9:1049. doi: 10.3389/fphys.2018.01049. eCollection 2018. Front Physiol. 2018. PMID: 30123137 Free PMC article. Review.
References
-
- Bailey CH, Giustetto M, Zhu H, Chen M, Kandel ER. A novel function for serotonin-mediated short-term facilitation in Aplysia: conversion of a transient, cell-wide homosynaptic hebbian plasticity into a persistent, protein synthesis-independent synapse-specific enhancement. Proc Natl Acad Sci USA 97: 11581–11586, 2000 - PMC - PubMed
-
- Ben-Chaim Y, Chanda B, Dascal N, Bezanilla F, Parnas I, Parnas H. Movement of “gating charge” is coupled to ligand binding in a G-protein-coupled receptor. Nature 444: 106–109, 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

