Diabetes mellitus induces bone marrow microangiopathy
- PMID: 20042708
- PMCID: PMC3548136
- DOI: 10.1161/ATVBAHA.109.200154
Diabetes mellitus induces bone marrow microangiopathy
Abstract
Objective: The impact of diabetes on the bone marrow (BM) microenvironment was not adequately explored. We investigated whether diabetes induces microvascular remodeling with negative consequence for BM homeostasis.
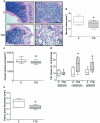
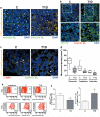
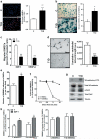
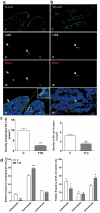
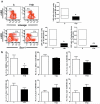
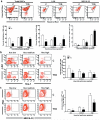
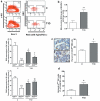
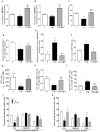
Methods and results: We found profound structural alterations in BM from mice with type 1 diabetes with depletion of the hematopoietic component and fatty degeneration. Blood flow (fluorescent microspheres) and microvascular density (immunohistochemistry) were remarkably reduced. Flow cytometry verified the depletion of MECA-32(+) endothelial cells. Cultured endothelial cells from BM of diabetic mice showed higher levels of oxidative stress, increased activity of the senescence marker beta-galactosidase, reduced migratory and network-formation capacities, and increased permeability and adhesiveness to BM mononuclear cells. Flow cytometry analysis of lineage(-) c-Kit(+) Sca-1(+) cell distribution along an in vivo Hoechst-33342 dye perfusion gradient documented that diabetes depletes lineage(-) c-Kit(+) Sca-1(+) cells predominantly in the low-perfused part of the marrow. Cell depletion was associated to increased oxidative stress, DNA damage, and activation of apoptosis. Boosting the antioxidative pentose phosphate pathway by benfotiamine supplementation prevented microangiopathy, hypoperfusion, and lineage(-) c-Kit(+) Sca-1(+) cell depletion.
Conclusions: We provide novel evidence for the presence of microangiopathy impinging on the integrity of diabetic BM. These discoveries offer the framework for mechanistic solutions of BM dysfunction in diabetes.
Figures
References
-
- Asahara T, Kawamoto A. Endothelial progenitor cells for postnatal vasculogenesis. Am J Physiol Cell Physiol. 2004;287:C572–579. - PubMed
-
- Dimmeler S. ATVB in focus: novel mediators and mechanisms in angiogenesis and vasculogenesis. Arterioscler Thromb Vasc Biol. 2005;25:2245. - PubMed
-
- Fischer C, Schneider M, Carmeliet P. Principles and therapeutic implications of angiogenesis, vasculogenesis and arteriogenesis. Handb Exp Pharmacol. 2006:157–212. - PubMed
-
- Jin DK, Shido K, Kopp HG, Petit I, Shmelkov SV, Young LM, Hooper AT, Amano H, Avecilla ST, Heissig B, Hattori K, Zhang F, Hicklin DJ, Wu Y, Zhu Z, Dunn A, Salari H, Werb Z, Hackett NR, Crystal RG, Lyden D, Rafii S. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nat Med. 2006;12:557–567. - PMC - PubMed
-
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

