Platelets and megakaryocytes contain functional nuclear factor-kappaB
- PMID: 20042710
- PMCID: PMC2853005
- DOI: 10.1161/ATVBAHA.109.197343
Platelets and megakaryocytes contain functional nuclear factor-kappaB
Abstract
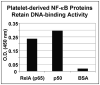
Objective: To investigate the presence and role of NF-kappaB proteins in megakaryocytes and platelets. The nuclear factor-kappaB (NF-kappaB) transcription factor family is well known for its role in eliciting inflammation and promoting cell survival. We discovered that human megakaryocytes and platelets express the majority of NF-kappaB family members, including the regulatory inhibitor-kappaB (I-kappaB) and I-kappa kinase (IKK) molecules.
Methods and results: Anucleate platelets exposed to NF-kappaB inhibitors demonstrated impaired fundamental functions involved in repairing vascular injury and thrombus formation. Specifically, NF-kappaB inhibition diminished lamellapodia formation, decreased clot retraction times, and reduced thrombus stability. Moreover, inhibition of I-kappaB-alpha phosphorylation (BAY-11-7082) reverted fully spread platelets back to a spheroid morphology. Addition of recombinant IKK-beta or I-kappaB-alpha protein to BAY inhibitor-treated platelets partially restored platelet spreading in I-kappaB-alpha inhibited platelets, and addition of active IKK-beta increased endogenous I-kappaB-alpha phosphorylation levels.
Conclusions: These novel findings support a crucial and nonclassical role for the NF-kappaB family in modulating platelet function and reveal that platelets are sensitive to NF-kappaB inhibitors. As NF-kappaB inhibitors are being developed as antiinflammatory and anticancer agents, they may have unintended effects on platelets. On the basis of these data, NF-kappaB is also identified as a new target to dampen unwanted platelet activation.
Figures





References
-
- Hagihara M, Higuchi A, Tamura N, Ueda Y, Hirabayashi K, Ikeda Y, Kato S, Sakamoto S, Hotta T, Handa S, Goto S. Platelets, after exposure to a high shear stress, induce IL-10-producing, mature dendritic cells in vitro. J Immunol. 2004;172:5297–5303. - PubMed
-
- von Hundelshausen P, Weber C. Platelets as immune cells: bridging inflammation and cardiovascular disease. Circ Res. 2007;100:27–40. - PubMed
-
- Gnatenko DV, Dunn JJ, McCorkle SR, Weissmann D, Perrotta PL, Bahou WF. Transcript profiling of human platelets using microarray and serial analysis of gene expression. Blood. 2003;101:2285–2293. - PubMed
-
- Denis MM, Tolley ND, Bunting M, Schwertz H, Jiang H, Lindemann S, Yost CC, Rubner FJ, Albertine KH, Swoboda KJ, Fratto CM, Tolley E, Kraiss LW, McIntyre TM, Zimmerman GA, Weyrich AS. Escaping the nuclear confines: signal-dependent pre-mRNA splicing in anucleate platelets. Cell. 2005;122:379–391. - PMC - PubMed
-
- Kieffer N, Guichard J, Farcet JP, Vainchenker W, Breton-Gorius J. Biosynthesis of major platelet proteins in human blood platelets. Eur J Biochem. 1987;164:189–195. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- RC1 HL100051/HL/NHLBI NIH HHS/United States
- DE011390/DE/NIDCR NIH HHS/United States
- ES01247/ES/NIEHS NIH HHS/United States
- P30 ES001247/ES/NIEHS NIH HHS/United States
- R01 HL095467/HL/NHLBI NIH HHS/United States
- R01 HL078603/HL/NHLBI NIH HHS/United States
- T32 DE007165/DE/NIDCR NIH HHS/United States
- HL078603/HL/NHLBI NIH HHS/United States
- T32-DE07165/DE/NIDCR NIH HHS/United States
- R21 HL086367/HL/NHLBI NIH HHS/United States
- HL086367/HL/NHLBI NIH HHS/United States
- R01 NS054578/NS/NINDS NIH HHS/United States
- HL095467/HL/NHLBI NIH HHS/United States
- R01 DE011390/DE/NIDCR NIH HHS/United States
- NS054578/NS/NINDS NIH HHS/United States
- RC1HL100051/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

