Activation of the cystic fibrosis transmembrane conductance regulator by the flavonoid quercetin: potential use as a biomarker of ΔF508 cystic fibrosis transmembrane conductance regulator rescue
- PMID: 20042712
- PMCID: PMC2970857
- DOI: 10.1165/rcmb.2009-0281OC
Activation of the cystic fibrosis transmembrane conductance regulator by the flavonoid quercetin: potential use as a biomarker of ΔF508 cystic fibrosis transmembrane conductance regulator rescue
Abstract
Therapies to correct the ΔF508 cystic fibrosis transmembrane conductance regulator (CFTR) folding defect require sensitive methods to detect channel activity in vivo. The β₂ adrenergic receptor agonists, which provide the CFTR stimuli commonly used in nasal potential difference assays, may not overcome the channel gating defects seen in ΔF508 CFTR after plasma membrane localization. In this study, we identify an agent, quercetin, that enhances the detection of surface ΔF508 CFTR, and is suitable for nasal perfusion. A screen of flavonoids in CFBE41o⁻ cells stably transduced with ΔF508 CFTR, corrected to the cell surface with low temperature growth, revealed that quercetin stimulated an increase in the short-circuit current. This increase was dose-dependent in both Fisher rat thyroid and CFBE41o⁻ cells. High concentrations inhibited Cl⁻ conductance. In CFBE41o⁻ airway cells, quercetin (20 μg/ml) activated ΔF508 CFTR, whereas the β₂ adrenergic receptor agonist isoproterenol did not. Quercetin had limited effects on cAMP levels, but did not produce detectable phosphorylation of the isolated CFTR R-domain, suggesting an activation independent of channel phosphorylation. When perfused in the nares of Cftr(+) mice, quercetin (20 μg/ml) produced a hyperpolarization of the potential difference that was absent in Cftr(-/-) mice. Finally, quercetin-induced, dose-dependent hyperpolarization of the nasal potential difference was also seen in normal human subjects. Quercetin activates CFTR-mediated anion transport in respiratory epithelia in vitro and in vivo, and may be useful in studies intended to detect the rescue of ΔF508 CFTR by nasal potential difference.
Figures
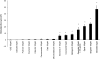
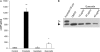
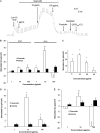
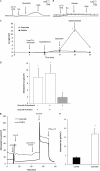
Similar articles
-
DeltaF508 CFTR processing correction and activity in polarized airway and non-airway cell monolayers.Pulm Pharmacol Ther. 2010 Aug;23(4):268-78. doi: 10.1016/j.pupt.2010.02.001. Epub 2010 Mar 10. Pulm Pharmacol Ther. 2010. PMID: 20226262 Free PMC article.
-
Deletion of phenylalanine 508 causes attenuated phosphorylation-dependent activation of CFTR chloride channels.J Physiol. 2000 May 1;524 Pt 3(Pt 3):637-48. doi: 10.1111/j.1469-7793.2000.00637.x. J Physiol. 2000. PMID: 10790148 Free PMC article.
-
Failure of cAMP agonists to activate rescued deltaF508 CFTR in CFBE41o- airway epithelial monolayers.J Physiol. 2005 Dec 1;569(Pt 2):601-15. doi: 10.1113/jphysiol.2005.096669. Epub 2005 Oct 6. J Physiol. 2005. PMID: 16210354 Free PMC article.
-
Selective activation of cystic fibrosis transmembrane conductance regulator Cl- and HCO3- conductances.JOP. 2001 Jul;2(4 Suppl):212-8. JOP. 2001. PMID: 11875262 Review.
-
CFTR chloride channel drug discovery--inhibitors as antidiarrheals and activators for therapy of cystic fibrosis.Curr Pharm Des. 2006;12(18):2235-47. doi: 10.2174/138161206777585148. Curr Pharm Des. 2006. PMID: 16787252 Review.
Cited by
-
CFTR Modulators: Shedding Light on Precision Medicine for Cystic Fibrosis.Front Pharmacol. 2016 Sep 5;7:275. doi: 10.3389/fphar.2016.00275. eCollection 2016. Front Pharmacol. 2016. PMID: 27656143 Free PMC article. Review.
-
Advances in Cystic Fibrosis Research in Qatar: A Commentary.J Pers Med. 2023 Feb 28;13(3):448. doi: 10.3390/jpm13030448. J Pers Med. 2023. PMID: 36983631 Free PMC article.
-
Suppression of CFTR premature termination codons and rescue of CFTR protein and function by the synthetic aminoglycoside NB54.J Mol Med (Berl). 2011 Nov;89(11):1149-61. doi: 10.1007/s00109-011-0787-6. Epub 2011 Jul 22. J Mol Med (Berl). 2011. PMID: 21779978 Free PMC article.
-
Virtual Drug Repositioning as a Tool to Identify Natural Small Molecules That Synergize with Lumacaftor in F508del-CFTR Binding and Rescuing.Int J Mol Sci. 2022 Oct 14;23(20):12274. doi: 10.3390/ijms232012274. Int J Mol Sci. 2022. PMID: 36293130 Free PMC article.
-
Fractional Exhalation Nitric Oxide (FeNO) changes in cystic fibrosis patients induced by compound honey syrup: a pretest-posttest clinical trial.BMC Pulm Med. 2023 Dec 5;23(1):488. doi: 10.1186/s12890-023-02787-9. BMC Pulm Med. 2023. PMID: 38053097 Free PMC article. Clinical Trial.
References
-
- Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med 2005;352:1992–2001. - PubMed
-
- Denning GM, Anderson MP, Amara JF, Marshall J, Smith AE, Welsh MJ. Processing of mutant cystic fibrosis transmembrane conductance regulator is temperature-sensitive. Nature 1992;358:761–764. - PubMed
-
- Egan ME, Glockner-Pagel J, Ambrose C, Cahill PA, Pappoe L, Balamuth N, Cho E, Canny S, Wagner CA, Geibel J, et al. Calcium-pump inhibitors induce functional surface expression of delta F508-CFTR protein in cystic fibrosis epithelial cells. Nat Med 2002;8:485–492. - PubMed
-
- Egan ME, Pearson M, Weiner SA, Rajendran V, Rubin D, Glockner-Pagel J, Canny S, Du K, Lukacs GL, Caplan MJ. Curcumin, a major constituent of turmeric, corrects cystic fibrosis defects. Science 2004;304:600–602. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

