Assessing lead time of selected ovarian cancer biomarkers: a nested case-control study
- PMID: 20042715
- PMCID: PMC2802285
- DOI: 10.1093/jnci/djp438
Assessing lead time of selected ovarian cancer biomarkers: a nested case-control study
Abstract
Background: CA125, human epididymis protein 4 (HE4), mesothelin, B7-H4, decoy receptor 3 (DcR3), and spondin-2 have been identified as potential ovarian cancer biomarkers. Except for CA125, their behavior in the prediagnostic period has not been evaluated.
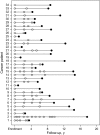
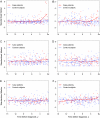
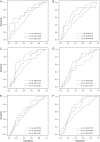
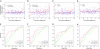
Methods: Immunoassays were used to determine concentrations of CA125, HE4, mesothelin, B7-H4, DcR3, and spondin-2 proteins in prediagnostic serum specimens (1-11 samples per participant) that were contributed 0-18 years before ovarian cancer diagnosis from 34 patients with ovarian cancer (15 with advanced-stage serous carcinoma) and during a comparable time interval before the reference date from 70 matched control subjects who were participating in the Carotene and Retinol Efficacy Trial. Lowess curves were fit to biomarker levels in cancer patients and control subjects separately to summarize mean levels over time. Receiver operating characteristic curves were plotted, and area-under-the curve (AUC) statistics were computed to summarize the discrimination ability of these biomarkers by time before diagnosis.
Results: Smoothed mean concentrations of CA125, HE4, and mesothelin (but not of B7-H4, DcR3, and spondin-2) began to increase (visually) in cancer patients relative to control subjects approximately 3 years before diagnosis but reached detectable elevations only within the final year before diagnosis. In descriptive receiver operating characteristic analyses, the discriminatory power of these biomarkers was limited (AUC statistics range = 0.56-0.75) but showed increasing accuracy with time approaching diagnosis (eg, AUC statistics for CA125 were 0.57, 0.68, and 0.74 for > or = 4, 2-4, and <2 years before diagnosis, respectively).
Conclusion: Serum concentrations of CA125, HE4, and mesothelin may provide evidence of ovarian cancer 3 years before clinical diagnosis, but the likely lead time associated with these markers appears to be less than 1 year.
Figures
Comment in
-
Designing early detection programs for ovarian cancer.J Natl Cancer Inst. 2010 Jan 6;102(1):3-4. doi: 10.1093/jnci/djp450. Epub 2009 Dec 30. J Natl Cancer Inst. 2010. PMID: 20042718 Free PMC article. No abstract available.
References
-
- Etzioni R, Urban N, Ramsey S, et al. The case for early detection. Nat Rev Cancer. 2003;3(4):243–252. - PubMed
-
- Jacobs IJ, Menon U. Progress and challenges in screening for early detection of ovarian cancer. Mol Cell Proteomics. 2004;3(4):355–366. - PubMed
-
- Bast RC, Urban N, Shridhar V, et al. Early detection of ovarian cancer: promise and reality. Cancer Treat Res. 2002;107:61–97. - PubMed
-
- Zurawski VR, Orjaseter H, Anderson A, Jellum E. Elevated serum CA 125 levels prior to diagnosis of ovarian neoplasia: relevance to early detection of ovarian cancer. Int J Cancer. 1988;42(5):667–680. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

