Dengue virus-induced hemorrhage in a nonhuman primate model
- PMID: 20042723
- PMCID: PMC2832810
- DOI: 10.1182/blood-2009-09-242990
Dengue virus-induced hemorrhage in a nonhuman primate model
Abstract
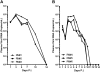
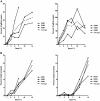
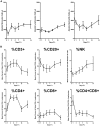
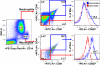
Lack of a dengue hemorrhagic animal model recapitulating human dengue virus infection has been a significant impediment in advancing our understanding of the early events involved in the pathogenesis of dengue disease. In efforts to address this issue, a group of rhesus macaques were intravenously infected with dengue virus serotype 2 (strain 16 681) at 1 x 10(7) PFU/animal. A classic dengue hemorrhage developed 3 to 5 days after infection in 6 of 6 animals. Blood chemistry appeared to be normal with exception of creatine phosphokinase, which peaked at 7 days after infection. A modest thrombocytopenia and noticeable neutropenia concomitant with slight decrease of hemoglobin and hematocrit were registered. In addition, the concentration of D-dimer was elevated significantly. Viremia peaked at 3 to 5 days after infection followed by an inverse relationship between T and B lymphocytes and a bimodal pattern for platelet-monocytes and platelet-neutrophil aggregates. Dengue virus containing platelets engulfed by monocytes was noted at 8 or 9 days after infection. Thus, rhesus macaques inoculated intravenously with a high dose of dengue virus produced dengue hemorrhage, which may provide a unique platform to define the early events in dengue virus infection and help identify which blood components contribute to the pathogenesis of dengue disease.
Figures

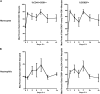
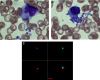
Similar articles
-
A non mouse-adapted dengue virus strain as a new model of severe dengue infection in AG129 mice.PLoS Negl Trop Dis. 2010 Apr 27;4(4):e672. doi: 10.1371/journal.pntd.0000672. PLoS Negl Trop Dis. 2010. PMID: 20436920 Free PMC article.
-
Dengue virus-specific CD4+ and CD8+ T lymphocytes target NS1, NS3 and NS5 in infected Indian rhesus macaques.Immunogenetics. 2012 Feb;64(2):111-21. doi: 10.1007/s00251-011-0566-0. Epub 2011 Sep 1. Immunogenetics. 2012. PMID: 21881953
-
Early Detection of Plasma Leakage in Dengue Hemorrhagic Fever.Acta Med Indones. 2018 Jul;50(3):183-184. Acta Med Indones. 2018. PMID: 30333266
-
Progress towards understanding the pathogenesis of dengue hemorrhagic fever.Virol Sin. 2017 Feb;32(1):16-22. doi: 10.1007/s12250-016-3855-9. Epub 2016 Nov 14. Virol Sin. 2017. PMID: 27853992 Free PMC article. Review.
-
Platelets in dengue infection: more than a numbers game.Platelets. 2022 Feb 17;33(2):176-183. doi: 10.1080/09537104.2021.1921722. Epub 2021 May 24. Platelets. 2022. PMID: 34027810 Review.
Cited by
-
A Cyclic Phosphoramidate Prodrug of 2'-Deoxy-2'-Fluoro-2'-C-Methylguanosine for the Treatment of Dengue Virus Infection.Antimicrob Agents Chemother. 2020 Nov 17;64(12):e00654-20. doi: 10.1128/AAC.00654-20. Print 2020 Nov 17. Antimicrob Agents Chemother. 2020. PMID: 32958712 Free PMC article.
-
Crosstalk between Platelets and SARS-CoV-2: Implications in Thrombo-Inflammatory Complications in COVID-19.Int J Mol Sci. 2023 Sep 15;24(18):14133. doi: 10.3390/ijms241814133. Int J Mol Sci. 2023. PMID: 37762435 Free PMC article. Review.
-
Platelets in Immune Response to Virus and Immunopathology of Viral Infections.Front Med (Lausanne). 2018 Apr 30;5:121. doi: 10.3389/fmed.2018.00121. eCollection 2018. Front Med (Lausanne). 2018. PMID: 29761104 Free PMC article. Review.
-
Molecular signatures associated with ZIKV exposure in human cortical neural progenitors.Nucleic Acids Res. 2016 Oct 14;44(18):8610-8620. doi: 10.1093/nar/gkw765. Epub 2016 Aug 31. Nucleic Acids Res. 2016. PMID: 27580721 Free PMC article.
-
Role of Platelet Cytokines in Dengue Virus Infection.Front Cell Infect Microbiol. 2020 Sep 30;10:561366. doi: 10.3389/fcimb.2020.561366. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33102253 Free PMC article. Review.
References
-
- Clark G, Gubler D, Rigau J. Dengue fever: CDC traveler's information on dengue fever. http://www.cdc.gov/travel/diseases/dengue.htm.
-
- Guzman M, Kouri G, Diaz M, et al. Dengue, one of the great emerging health challenges of the 21st century. Expert Rev Vaccines. 2004;3(5):511–520. - PubMed
-
- Chang SF, Huang JH, Chen LK, et al. Retrospective serological study on sequential dengue virus serotypes 1 to 4 epidemics in Tainan City, Taiwan, 1994 to 2000. J Microbiol Immunol Infect. 2008;41(5):377–385. - PubMed
-
- Chan KP, Lau GK, Doraisingham S, Chan YC. Adult dengue deaths in Singapore. Clin Diagn Virol. 1995;4(3):213–222. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

