A sensitive period of mice inhibitory system to neonatal GABA enhancement by vigabatrin is brain region dependent
- PMID: 20043003
- PMCID: PMC3055404
- DOI: 10.1038/npp.2009.219
A sensitive period of mice inhibitory system to neonatal GABA enhancement by vigabatrin is brain region dependent
Abstract
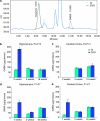
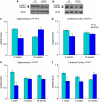
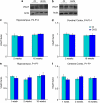
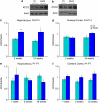
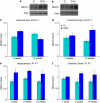
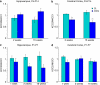
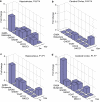
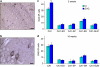
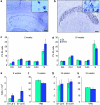
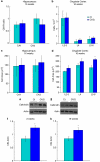
Neurodevelopmental disorders, such as schizophrenia and autism, have been associated with disturbances of the GABAergic system in the brain. We examined immediate and long-lasting influences of exposure to the GABA-potentiating drug vigabatrin (GVG) on the GABAergic system in the hippocampus and cerebral cortex, before and during the developmental switch in GABA function (postnatal days P1-7 and P4-14). GVG induced a transient elevation of GABA levels. A feedback response to GABA enhancement was evident by a short-term decrease in glutamate decarboxylase (GAD) 65 and 67 levels. However, the number of GAD65/67-immunoreactive (IR) cells was greater in 2-week-old GVG-treated mice. A long-term increase in GAD65 and GAD67 levels was dependent on brain region and treatment period. Vesicular GABA transporter was insensitive to GVG. The overall effect of GVG on the Cl(-) co-transporters NKCC1 and KCC2 was an enhancement of their synthesis, which was dependent on the treatment period and brain region studied. In addition, a short-term increase was followed by a long-term decrease in KCC2 oligomerization in the cell membrane of P4-14 hippocampi and cerebral cortices. Analysis of the Ca(2+) binding proteins expressed in subpopulations of GABAergic cells, parvalbumin and calbindin, showed region-specific effects of GVG during P4-14 on parvalbumin-IR cell density. Moreover, calbindin levels were elevated in GVG mice compared to controls during this period. Cumulatively, these results suggest a particular susceptibility of the hippocampus to GVG when exposed during days P4-14. In conclusion, our studies have identified modifications of key components in the inhibitory system during a critical developmental period. These findings provide novel insights into the deleterious consequences observed in children following prenatal and neonatal exposure to GABA-potentiating drugs.
Figures

Similar articles
-
Long-lasting glutamatergic modulation induced by neonatal GABA enhancement in mice.Neuropharmacology. 2014 Apr;79:616-25. doi: 10.1016/j.neuropharm.2013.12.015. Epub 2014 Jan 21. Neuropharmacology. 2014. PMID: 24462620
-
Impaired synaptogenesis and long-term modulation of behavior following postnatal elevation of GABA levels in mice.Neuropharmacology. 2008 Feb;54(2):387-98. doi: 10.1016/j.neuropharm.2007.10.012. Epub 2007 Dec 11. Neuropharmacology. 2008. PMID: 18063001
-
Expression of GABA signaling molecules KCC2, NKCC1, and GAD1 in cortical development and schizophrenia.J Neurosci. 2011 Jul 27;31(30):11088-95. doi: 10.1523/JNEUROSCI.1234-11.2011. J Neurosci. 2011. PMID: 21795557 Free PMC article.
-
Experimental studies and controlled clinical testing of valproate and vigabatrin.Acta Neurol Scand. 1988 Oct;78(4):241-70. doi: 10.1111/j.1600-0404.1988.tb03655.x. Acta Neurol Scand. 1988. PMID: 3146860 Review.
-
Chloride transporters and GABA polarity in developmental, neurological and psychiatric conditions.Neurosci Biobehav Rev. 2018 Jul;90:260-271. doi: 10.1016/j.neubiorev.2018.05.001. Epub 2018 May 2. Neurosci Biobehav Rev. 2018. PMID: 29729285 Review.
Cited by
-
Neurobehavioral effects of vigabatrin and its ability to induce DNA damage in brain cells after acute treatment in rats.Psychopharmacology (Berl). 2017 Jan;234(1):129-136. doi: 10.1007/s00213-016-4446-z. Epub 2016 Sep 27. Psychopharmacology (Berl). 2017. PMID: 27678549
-
Altered behavioral development in Nrf2 knockout mice following early postnatal exposure to valproic acid.Brain Res Bull. 2014 Oct;109:132-42. doi: 10.1016/j.brainresbull.2014.10.006. Epub 2014 Oct 20. Brain Res Bull. 2014. PMID: 25454122 Free PMC article.
-
Enhanced GIRK2 channel signaling in Down syndrome: A feasible role in the development of abnormal nascent neural circuits.Front Genet. 2022 Sep 12;13:1006068. doi: 10.3389/fgene.2022.1006068. eCollection 2022. Front Genet. 2022. PMID: 36171878 Free PMC article. Review.
-
Gender-specific effect of Mthfr genotype and neonatal vigabatrin interaction on synaptic proteins in mouse cortex.Neuropsychopharmacology. 2011 Jul;36(8):1714-28. doi: 10.1038/npp.2011.52. Epub 2011 Apr 13. Neuropsychopharmacology. 2011. PMID: 21490592 Free PMC article.
-
Neurodevelopment, GABA system dysfunction, and schizophrenia.Neuropsychopharmacology. 2015 Jan;40(1):190-206. doi: 10.1038/npp.2014.95. Epub 2014 Apr 24. Neuropsychopharmacology. 2015. PMID: 24759129 Free PMC article. Review.
References
-
- Aguado F, Carmona MA, Pozas E, Aguiló A, Martínez-Guijarro FJ, Alcantara S, et al. BDNF regulates spontaneous correlated activity at early developmental stages by increasing synaptogenesis, expression of the K+/Cl− co-transporter KCC2. Development. 2003;130:1267–1280. - PubMed
-
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity. Neurosci Biobehav Rev. 2003;27:3–18. - PubMed
-
- Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

