TDP-43 dimerizes in human cells in culture
- PMID: 20043239
- PMCID: PMC11498789
- DOI: 10.1007/s10571-009-9489-9
TDP-43 dimerizes in human cells in culture
Abstract
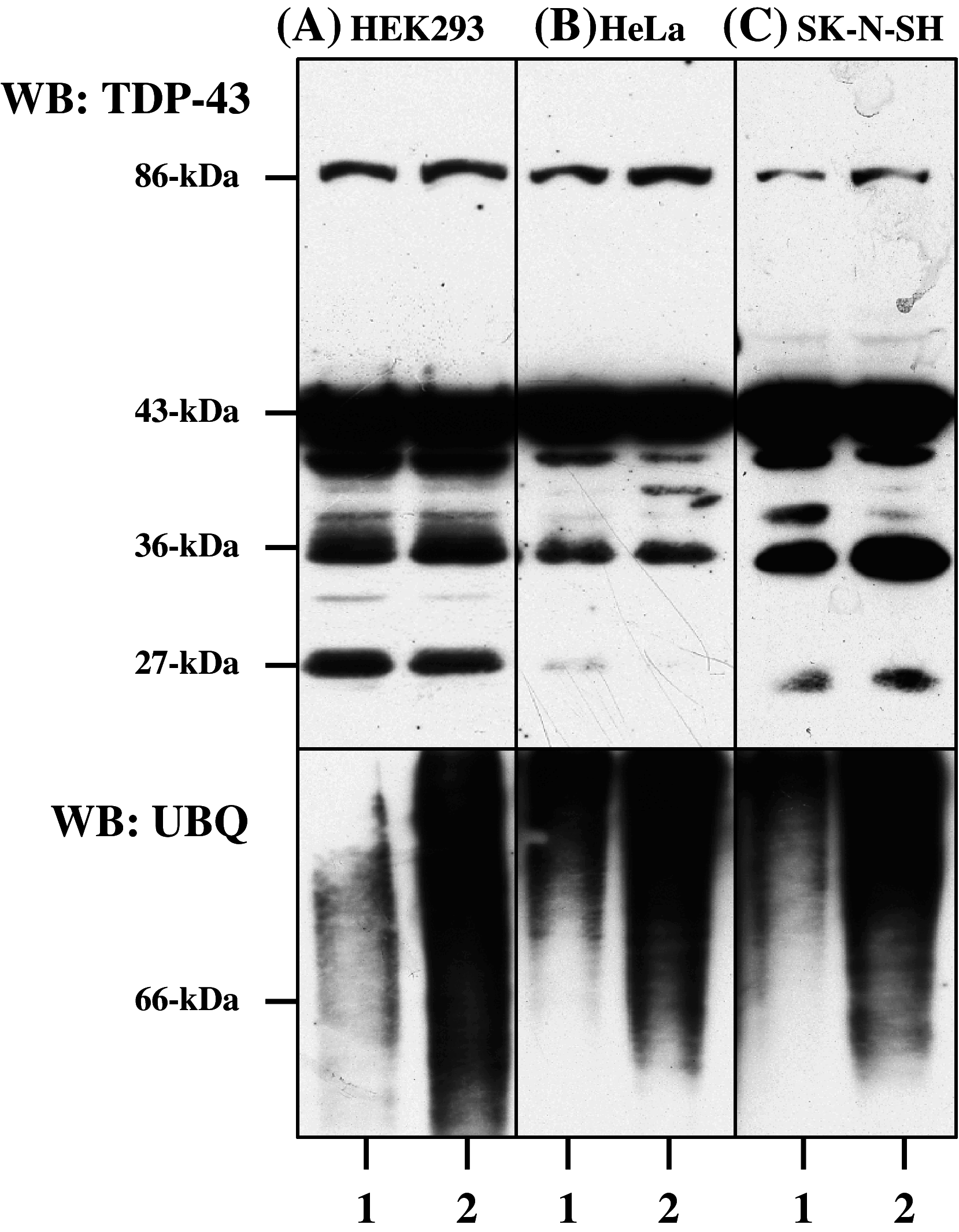
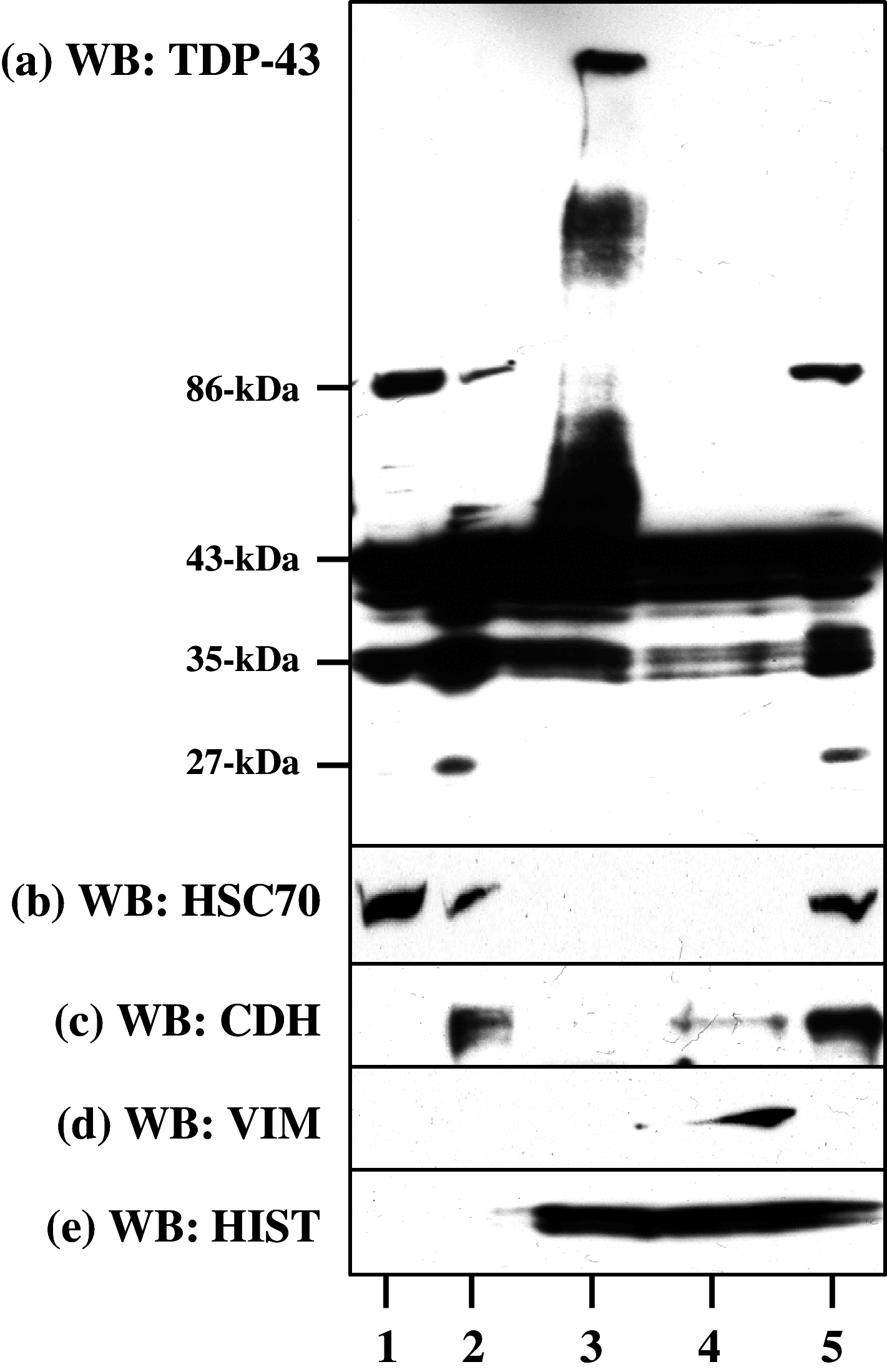
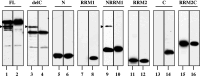
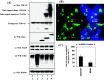
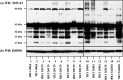
TAR DNA-binding protein-43 (TDP-43) is a 43-kDa nuclear protein involved in regulation of gene expression. Abnormally, phosphorylated, ubiquitinated, and aggregated TDP-43 constitute a principal component of neuronal and glial cytoplasmic and nuclear inclusions in the brains of frontotemporal lobar degeneration with ubiquitin-positive inclusions (FTLD-U) and amyotrophic lateral sclerosis (ALS), although the molecular mechanism that triggers aggregate formation remains unknown. By Western blot analysis using anti-TDP-43 antibodies, we identified a band with an apparent molecular mass of 86-kDa in HEK293, HeLa, and SK-N-SH cells in culture. It was labeled with both N-terminal-specific and C-terminal-specific TDP-43 antibodies, enriched in the cytosolic fraction, and the expression levels were reduced by TDP-43 siRNA but unaltered by treatment with MG-132 or by expression of ubiqulin-1 or casein kinase-1. By immunoprecipitation analysis, we found the interaction between the endogenous full-length TDP-43 and the exogenous Flag-tagged TDP-43, and identified the N-terminal half of TDP-43 spanning amino acid residues 3-183 as an intermolecular interaction domain. When the tagged 86-kDa tandemly connected dimer of TDP-43 was overexpressed in HEK293, it was sequestered in the cytoplasm and promoted an accumulation of high-molecular-mass TDP-43-immunoreactive proteins. Furthermore, the 86-kDa band was identified in the immunoblot of human brain tissues, including those of ALS. These results suggest that the 86-kDa band represents dimerized TDP-43 expressed constitutively in normal cells under physiological conditions.
Figures


Similar articles
-
Truncation and pathogenic mutations facilitate the formation of intracellular aggregates of TDP-43.Hum Mol Genet. 2009 Sep 15;18(18):3353-64. doi: 10.1093/hmg/ddp275. Epub 2009 Jun 10. Hum Mol Genet. 2009. PMID: 19515851
-
Conserved acidic amino acid residues in a second RNA recognition motif regulate assembly and function of TDP-43.PLoS One. 2012;7(12):e52776. doi: 10.1371/journal.pone.0052776. Epub 2012 Dec 26. PLoS One. 2012. PMID: 23300771 Free PMC article.
-
Expression of TDP-43 C-terminal Fragments in Vitro Recapitulates Pathological Features of TDP-43 Proteinopathies.J Biol Chem. 2009 Mar 27;284(13):8516-24. doi: 10.1074/jbc.M809462200. Epub 2009 Jan 21. J Biol Chem. 2009. PMID: 19164285 Free PMC article.
-
[The molecular mechanisms of intracellular TDP-43 aggregates].Brain Nerve. 2009 Nov;61(11):1292-300. Brain Nerve. 2009. PMID: 19938686 Review. Japanese.
-
[Significance of the TDP-43 deposition in FTLD-U and ALS].Rinsho Shinkeigaku. 2008 Nov;48(11):994-7. doi: 10.5692/clinicalneurol.48.994. Rinsho Shinkeigaku. 2008. PMID: 19198142 Review. Japanese.
Cited by
-
Multi-phaseted problems of TDP-43 in selective neuronal vulnerability in ALS.Cell Mol Life Sci. 2021 May;78(10):4453-4465. doi: 10.1007/s00018-021-03792-z. Epub 2021 Mar 11. Cell Mol Life Sci. 2021. PMID: 33709256 Free PMC article. Review.
-
The Neurotoxic TAU45-230 Fragment Accumulates in Upper and Lower Motor Neurons in Amyotrophic Lateral Sclerosis Subjects.Mol Med. 2016 Oct;22:477-486. doi: 10.2119/molmed.2016.00095. Epub 2016 Aug 3. Mol Med. 2016. PMID: 27496042 Free PMC article.
-
RBM45 homo-oligomerization mediates association with ALS-linked proteins and stress granules.Sci Rep. 2015 Sep 22;5:14262. doi: 10.1038/srep14262. Sci Rep. 2015. PMID: 26391765 Free PMC article.
-
Inhibiting glycogen synthase kinase 3 suppresses TDP-43-mediated neurotoxicity in a caspase-dependent manner.Res Sq [Preprint]. 2025 May 29:rs.3.rs-6527592. doi: 10.21203/rs.3.rs-6527592/v1. Res Sq. 2025. PMID: 40502782 Free PMC article. Preprint.
-
The extreme N-terminus of TDP-43 mediates the cytoplasmic aggregation of TDP-43 and associated toxicity in vivo.Brain Res. 2016 Sep 15;1647:57-64. doi: 10.1016/j.brainres.2016.04.069. Epub 2016 May 4. Brain Res. 2016. PMID: 27155453 Free PMC article. Review.
References
-
- Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, Oda T (2006) TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun 351:602–611 - PubMed
-
- Ayala YM, Pantano S, D’Ambrogio A, Buratti E, Brindisi A, Marchetti C, Romano M, Baralle FE (2005) Human, Drosophila, and C. elegans TDP43: nucleic acid binding properties and splicing regulatory function. J Mol Biol 348:575–588 - PubMed
-
- Ayala YM, Zago P, D’Ambrogio A, Xu YF, Petrucelli L, Buratti E, Baralle FE (2008) Structural determinants of the cellular localization and shuttling of TDP-43. J Cell Sci 121:3778–3785 - PubMed
-
- Buratti E, Baralle FE (2008) Multiple roles of TDP-43 in gene expression, splicing regulation, and human disease. Front Biosci 13:867–878 - PubMed
-
- Buratti E, Brindisi A, Giombi M, Tisminetzky S, Ayala YM, Baralle FE (2005) TDP-43 binds heterogeneous nuclear ribonucleoprotein A/B through its C-terminal tail: an important region for the inhibition of cystic fibrosis transmembrane conductance regulator exon 9 splicing. J Biol Chem 280:37572–37584 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

