Alterations in intracellular calcium ion concentrations in cerebellar granule cells of the CACNA1A mutant mouse, leaner, during postnatal development
- PMID: 20043243
- PMCID: PMC2921553
- DOI: 10.1007/s12640-009-9147-5
Alterations in intracellular calcium ion concentrations in cerebellar granule cells of the CACNA1A mutant mouse, leaner, during postnatal development
Abstract
Maintaining calcium ion (Ca²+) homeostasis is crucial for normal neuronal function. Altered Ca²+ homeostasis interferes with Ca²+ signaling processes and affects neuronal survival. In this study, we used homozygous leaner and tottering mutant mice, which carry autosomal recessive mutations in the gene coding for the α(1A) pore forming subunit of Ca(V)2.1 (P/Q-type) voltage-gated calcium channels (VGCC). Leaner mice show severe ataxia and epilepsy, while tottering mice are less severely affected. Leaner cerebellar granule cells (CGC) show extensive apoptotic cell death that peaks at postnatal (P) day 20 and continues into adulthood. Intracellular Ca²+ ([Ca²+](i)) concentrations in leaner and tottering mouse Purkinje cells have been described, but [Ca²+](i) concentrations have not been reported for granule cells, the largest neuronal population of the cerebellum. Using the ratiometric dye, Fura-2 AM, we investigated the role of Ca²+ homeostasis in CGC death during postnatal development by demonstrating basal [Ca²+](i), depolarization induced Ca²+ transients, and Ca²+ transients after completely blocking Ca(V)2.1 VGCC. From P20 onward, basal [Ca²+](i) levels in leaner CGC were significantly lower compared to age-matched wild-type CGC. We also compared basal [Ca²+](i) levels in leaner and wild-type CGC to basal [Ca²+](i) in tottering CGC. Potassium chloride induced depolarization revealed no significant difference in Ca²+ transients between leaner and wild-type CGC, indicating that even though leaner CGC have dysfunctional P/Q-type VGCC, Ca²+ transients after depolarization are the same. This suggests that other VGCC are compensating for the dysfunctional P/Q channels. This finding was further confirmed by completely blocking Ca(V)2.1 VGCC using ω-Agatoxin IV-A.
Figures
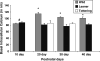
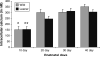
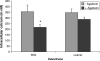
References
-
- Bawa B, Abbott LC. Analysis of calcium ion homeostasis and mitochondrial function in cerebellar granule cells of adult CaV2.1 calcium ion channel mutant mice. Neurotox Res. 2008;13(1):1–18. - PubMed
-
- Etheredge JA, Murchison D, Abbott LC, Griffith WH. Functional compensation by other voltage-gated Ca2+ channels in mouse basal forebrain neurons with Ca(V)2.1 mutations. Brain Res. 2007;1140:105–119. - PubMed
-
- Fletcher CF, Lutz CM, O'Sullivan TN, Shaughnessy JD, Jr, Hawkes R, Frankel WN, Copeland NG, Jenkins NA. Absence epilepsy in tottering mutant mice is associated with calcium channel defects. Cell. 1996;87:607–617. - PubMed
-
- Lau FC, Frank TC, Nahm S-S, Stoica G, Abbott LC. Postnatal apoptosis in cerebellar granule cells of homozygous leaner (tg1a/tg1a) mice. Neurotox Res. 2004;6(4):267–280. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

