ConteXt of change--X inactivation and disease
- PMID: 20043281
- PMCID: PMC3377189
- DOI: 10.1002/emmm.200900053
ConteXt of change--X inactivation and disease
Abstract
Epigenetic regulation is important for stable maintenance of cell identity. For continued function of organs and tissues, illegitimate changes in cell identity must be avoided. Failure to do so can trigger tumour development and disease. How epigenetic patterns are established during cell differentiation has been explored by studying model systems such as X inactivation. Mammals balance the X-linked gene dosage between the sexes by silencing of one of the two X chromosomes in females. This is initiated by expression of the non-coding X-inactive specific transcript (Xist) RNA and depends on specific cellular contexts, in which essential silencing factors are expressed. Normally X inactivation is initiated in early embryogenesis, but recent reports identified instances where Xist is expressed and can initiate gene repression. Here we describe the features that characterize the cellular permissivity to initiation of X inactivation and note that these can also occur in cancer cells and in specific haematopoietic progenitors. We propose that embryonic pathways for epigenetic regulation are re-established in adult progenitor cells and tumour cells. Understanding their reactivation will deepen our understanding of tumourigenesis and may be exploited for cancer therapy.
Figures
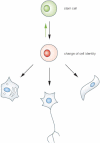

It is thought that the mammalian sex chromosomes X and Y originated from an autosome pair, when a spontaneous mutation arose specifying male sex (blue line). Evolution of the sex chromosomes led to the loss of sequences from the Y chromosome leading to a gene dosage difference between XY males and XX females.
For balancing gene dosage between the sexes random inactivation of one of the two X chromosomes in female cells is initiated by Xist (pink) and maintained by other mechanisms in differentiated cells (black).
Experimental expression of Xist in male cells can be used for probing the epigenetic context. In cells expressing initiation factors, Xist mediated gene silencing is initiated and loss of X-linked gene expression then causes the death of the cells. Therefore, a phenotype can be observed depending on the epigenetic context of the cell.


Tumours can develop from progenitors that provide an epigenetic context for the establishment of epigenetic patterns, which can be identified by an active gene silencing pathway. Aggressive T-cell lymphomas provide an example, as they maintain properties of their T-cell progenitor origin such as SATB1 expression.
Other tumours are thought to establish a similar cellular context when they progress to malignant disease. Breast cancer cells provide an example for this type of tumour. When breast cancers progress to a more malignant and highly metastatic phenotype, SATB1 expression can be elevated. This might go along with a reprogramming of the tumour cell transcriptome, and albeit direct evidence is still awaited, with the re-establishment of pathways for gene silencing. In breast cancer SATB1 expression is unrelated to the cellular context from which the tumour originated and is acquired at a late stage in tumourigenesis. Establishing a progenitor-like context might endow tumour cells with developmental pathways that contribute to metastasis.
Similar articles
-
Cancer progenitors and epigenetic contexts: an Xisting connection.Epigenetics. 2009 Nov 16;4(8):568-70. doi: 10.4161/epi.4.8.10186. Epub 2009 Nov 27. Epigenetics. 2009. PMID: 19923898 Review.
-
SATB1 defines the developmental context for gene silencing by Xist in lymphoma and embryonic cells.Dev Cell. 2009 Apr;16(4):507-16. doi: 10.1016/j.devcel.2009.03.006. Dev Cell. 2009. PMID: 19386260 Free PMC article.
-
Sex-specific silencing of X-linked genes by Xist RNA.Proc Natl Acad Sci U S A. 2016 Jan 19;113(3):E309-18. doi: 10.1073/pnas.1515971113. Epub 2016 Jan 6. Proc Natl Acad Sci U S A. 2016. PMID: 26739568 Free PMC article.
-
Eutherian mammals use diverse strategies to initiate X-chromosome inactivation during development.Nature. 2011 Apr 21;472(7343):370-4. doi: 10.1038/nature09872. Epub 2011 Apr 6. Nature. 2011. PMID: 21471966
-
X chromosome inactivation: silencing, topology and reactivation.Curr Opin Cell Biol. 2017 Jun;46:54-61. doi: 10.1016/j.ceb.2017.01.007. Epub 2017 Feb 23. Curr Opin Cell Biol. 2017. PMID: 28236732 Review.
Cited by
-
Regulation of DNA replication timing on human chromosome by a cell-type specific DNA binding protein SATB1.PLoS One. 2012;7(8):e42375. doi: 10.1371/journal.pone.0042375. Epub 2012 Aug 7. PLoS One. 2012. PMID: 22879953 Free PMC article.
-
The inactive X chromosome is epigenetically unstable and transcriptionally labile in breast cancer.Genome Res. 2015 Apr;25(4):488-503. doi: 10.1101/gr.185926.114. Epub 2015 Feb 4. Genome Res. 2015. PMID: 25653311 Free PMC article.
-
X-factors in human disease: impact of gene content and dosage regulation.Hum Mol Genet. 2021 Oct 1;30(R2):R285-R295. doi: 10.1093/hmg/ddab221. Hum Mol Genet. 2021. PMID: 34387327 Free PMC article. Review.
-
The X chromosome and sex-specific effects in infectious disease susceptibility.Hum Genomics. 2019 Jan 8;13(1):2. doi: 10.1186/s40246-018-0185-z. Hum Genomics. 2019. PMID: 30621780 Free PMC article. Review.
-
Oncogenic potential of yin yang 1 mediated through control of imprinted genes.Crit Rev Oncog. 2011;16(3-4):199-209. doi: 10.1615/critrevoncog.v16.i3-4.40. Crit Rev Oncog. 2011. PMID: 22248054 Free PMC article. Review.
References
-
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. RETT syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–188. - PubMed
-
- Anderson-Cohen M, Holland SM, Kuhns DB, Fleisher TA, Ding L, Brenner S, Malech HL, Roesler J. Severe phenotype of chronic granulomatous disease presenting in a female with a de novo mutation in gp91-phox and a non familial, extremely skewed X chromosome inactivation. Clin Immunol (Orlando, FL) 2003;109:308–317. - PubMed
-
- Archer H, Evans J, Leonard H, Colvin L, Ravine D, Christodoulou J, Williamson S, Charman T, Bailey ME, Sampson J, et al. Correlation between clinical severity in patients with RETT syndrome with a p.R168X or p.T158M MECP2 mutation, and the direction and degree of skewing of X-chromosome inactivation. J Med Genet. 2007;44:148–152. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

