Telomerase enzyme inhibition (TEI) and cytolytic therapy in the management of androgen independent osseous metastatic prostate cancer
- PMID: 20043297
- PMCID: PMC3910097
- DOI: 10.1002/pros.21096
Telomerase enzyme inhibition (TEI) and cytolytic therapy in the management of androgen independent osseous metastatic prostate cancer
Abstract
Background: Recurrent prostate cancer can be osseous, androgen independent and lethal. The purpose is to discern the efficacy of synthetic small molecule telomerase enzyme inhibitors (TEI) alone or in combination with other cytotoxic therapies in controlling metastatic osseous prostate cancer.
Methods: C4-2B was pre-treated with a match or mismatch TEI for 6 weeks and then inoculated into nude mice subcutaneously or intraosseously. In a separate experiment, untreated C4-2B was injected into femur of nude mice. The mice were divided into seven systemic "combination" treatment groups of control, Ad-BSP-E1a virus, docetaxel, mismatch and match TEI. Serum PSA was followed longitudinally. Histology analyses and histomorphometry were performed. Repeated measure analysis was applied for statistical analysis and Bonferroni method was used in multiple comparisons.
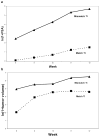
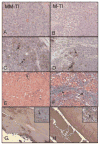
Results: In the pre-treated study, the PSA of match treated cells in subcutaneous or intraosseous model was significantly lower than mismatch TEI or PBS treated group (P < 0.05). Histology revealed increased fibrosis, apoptosis and decreased PSA staining in the match TEI treated subcutaneous xenografts. In the combination treatment study, the PSA was significantly lower in single/double treatment and triple treatment than control (P < 0.05). Histology revealed that triple therapy mice had normal femur architecture. Histomorphometrics revealed that the area of femur tumor and woven bone was significantly positively correlated (P = 0.007).
Conclusions: Multiple lines of data point toward the efficacy of systemically administered telomerase inhibitors. Combining cytotoxic regimens with telomerase inhibitors could be beneficial in controlling prostate cancer. Clinical trials are warranted to explore the efficacy of TEI in prostate cancer.
Figures




Similar articles
-
Small molecule, oligonucleotide-based telomerase template inhibition in combination with cytolytic therapy in an in vitro androgen-independent prostate cancer model.Urol Oncol. 2006 Mar-Apr;24(2):141-51. doi: 10.1016/j.urolonc.2005.11.003. Urol Oncol. 2006. PMID: 16520278
-
Conditionally replicating adenovirus therapy utilizing bone sialoprotein promoter (Ad-BSP-E1a) in an in vivo study of treating androgen-independent intraosseous prostate cancer.Urol Oncol. 2011 Nov-Dec;29(6):624-33. doi: 10.1016/j.urolonc.2009.08.012. Epub 2009 Dec 6. Urol Oncol. 2011. PMID: 19963408
-
Effective treatment of experimental androgen sensitive and androgen independent intraosseous prostate cancer with targeted cytotoxic somatostatin analogue AN-238.J Urol. 2004 Feb;171(2 Pt 1):911-5. doi: 10.1097/01.ju.0000105101.77884.06. J Urol. 2004. PMID: 14713852
-
Management of androgen-independent prostate cancer.Cancer Control. 2004 Nov-Dec;11(6):364-73. doi: 10.1177/107327480401100604. Cancer Control. 2004. PMID: 15625524 Review.
-
P450-dependent enzymes as targets for prostate cancer therapy.J Steroid Biochem Mol Biol. 1996 Jan;56(1-6 Spec No):133-43. doi: 10.1016/0960-0760(95)00230-8. J Steroid Biochem Mol Biol. 1996. PMID: 8603034 Review.
Cited by
-
Pharmacological inhibition of noncanonical EED-EZH2 signaling overcomes chemoresistance in prostate cancer.Theranostics. 2021 May 8;11(14):6873-6890. doi: 10.7150/thno.49235. eCollection 2021. Theranostics. 2021. PMID: 34093859 Free PMC article.
-
Knockdown of tankyrase 1 inhibits the progression of gastric adenocarcinoma via regulating human telomerase reverse transcriptase and telomeric repeat binding factor 1.J Gastrointest Oncol. 2022 Apr;13(2):559-568. doi: 10.21037/jgo-22-82. J Gastrointest Oncol. 2022. PMID: 35557584 Free PMC article.
-
Telomeres and telomerase: from discovery to clinical trials.Chem Biol. 2009 Dec 24;16(12):1219-23. doi: 10.1016/j.chembiol.2009.12.001. Chem Biol. 2009. PMID: 20064431 Free PMC article.
-
High fat diet increases melanoma cell growth in the bone marrow by inducing osteopontin and interleukin 6.Oncotarget. 2016 May 3;7(18):26653-69. doi: 10.18632/oncotarget.8474. Oncotarget. 2016. PMID: 27049717 Free PMC article.
References
-
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):228. - PubMed
-
- Miller DC, Hafez KS, Stewart A, Montie JE, Wei JT. Prostate carcinoma presentation, diagnosis, and staging: An update from the National Cancer Data Base. Cancer. 2003;98(6):1169–1178. - PubMed
-
- Roehl KA, Han M, Ramos CG, Antenor JA, Catalona WJ. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: Long-term results. J Urol. 2004;172(3):910–914. - PubMed
-
- Han M, Partin AW, Pound CR, Epstein JI, Walsh PC. Long-term biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy. The 15-year Johns Hopkins experience. Urol Clin North Am. 2001;28(3):555–565. - PubMed
-
- Perez CA, Michalski JM, Lockett MA. Chemical disease-free survival in localized carcinoma of prostate treated with external beam irradiation: Comparison of American Society of Therapeutic Radiology and Oncology Consensus or 1 ng/mL as endpoint. Int J Radiat Oncol Biol Phys. 2001;49(5):1287–1296. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

