Vitamin D receptor ligands, adenomatous polyposis coli, and the vitamin D receptor FokI polymorphism collectively modulate beta-catenin activity in colon cancer cells
- PMID: 20043299
- PMCID: PMC3074190
- DOI: 10.1002/mc.20603
Vitamin D receptor ligands, adenomatous polyposis coli, and the vitamin D receptor FokI polymorphism collectively modulate beta-catenin activity in colon cancer cells
Abstract
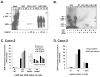
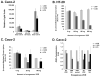
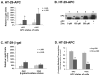
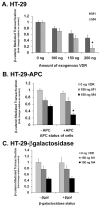
The activity of beta-catenin, commonly dysregulated in human colon cancers, is inhibited by the vitamin D receptor (VDR), and this mechanism is postulated to explain the putative anti-cancer activity of vitamin D metabolites in the colon. We investigated the effect of a common FokI restriction site polymorphism (F/f) in the human VDR gene as well as the effect of anti-tumorigenic 1,25-dihydroxyvitamin D(3) (1,25D) and pro-tumorigenic lithocholic acid (LCA) VDR ligands on beta-catenin transcriptional activity. Furthermore, the influence of a major regulatory protein of beta-catenin, the APC tumor suppressor gene, on VDR-dependent inhibition of beta-catenin activity was examined. We report herein that beta-catenin-mediated transcription is most effectively suppressed by the VDR FokI variant F/M4 when 1,25D is limiting. Using Caco-2 colorectal cancer (CRC) cells, it was observed that VDR ligands, 1,25D and LCA, both suppress beta-catenin transcriptional activity, though 1,25D exhibited significantly greater inhibition. Moreover, 1,25D, but not LCA, suppressed endogenous expression of the beta-catenin target gene DKK-4 independent of VDR DNA-binding activity. These results support beta-catenin sequestration away from endogenous gene targets by 1,25D-VDR. This activity is most efficiently mediated by the FokI gene variant F/M4, a VDR allele previously associated with protection against CRC. Interestingly, we found the inhibition of beta-catenin activity by 1,25D-VDR was significantly enhanced by wild-type APC. These results reveal a previously unrecognized role for 1,25D-VDR in APC/beta-catenin cross talk. Collectively, these findings strengthen evidence favoring a direct effect on the Wnt-signaling molecule beta-catenin as one anti-cancer target of 1,25D-VDR action in the colorectum.
Figures

Similar articles
-
A role for the vitamin D pathway in non-intestinal lesions in genetic and carcinogen models of colorectal cancer and in familial adenomatous polyposis.Oncotarget. 2016 Dec 6;7(49):80508-80520. doi: 10.18632/oncotarget.12768. Oncotarget. 2016. PMID: 27768599 Free PMC article.
-
Vitamin D and Wnt/beta-catenin pathway in colon cancer: role and regulation of DICKKOPF genes.Anticancer Res. 2008 Sep-Oct;28(5A):2613-23. Anticancer Res. 2008. PMID: 19035286 Review.
-
Vitamin D receptor deficiency enhances Wnt/β-catenin signaling and tumor burden in colon cancer.PLoS One. 2011;6(8):e23524. doi: 10.1371/journal.pone.0023524. Epub 2011 Aug 15. PLoS One. 2011. PMID: 21858154 Free PMC article.
-
The inhibition of Wnt/beta-catenin signalling by 1alpha,25-dihydroxyvitamin D3 is abrogated by Snail1 in human colon cancer cells.Endocr Relat Cancer. 2007 Mar;14(1):141-51. doi: 10.1677/ERC-06-0028. Endocr Relat Cancer. 2007. PMID: 17395983
-
The effect of 1,25 dihydroxyvitamin D3 treatment on the mRNA levels of β catenin target genes in mice with colonic inactivation of both APC alleles.J Steroid Biochem Mol Biol. 2015 Apr;148:103-10. doi: 10.1016/j.jsbmb.2015.01.009. Epub 2015 Jan 15. J Steroid Biochem Mol Biol. 2015. PMID: 25597951 Free PMC article. Review.
Cited by
-
Nutrigenomic underpinnings of intestinal stem cells in inflammatory bowel disease and colorectal cancer development.Front Genet. 2024 Aug 30;15:1349717. doi: 10.3389/fgene.2024.1349717. eCollection 2024. Front Genet. 2024. PMID: 39280096 Free PMC article. Review.
-
Role of the RANK/RANKL/OPG and Wnt/β-Catenin Systems in CKD Bone and Cardiovascular Disorders.Calcif Tissue Int. 2021 Apr;108(4):439-451. doi: 10.1007/s00223-020-00803-2. Epub 2021 Feb 13. Calcif Tissue Int. 2021. PMID: 33586001 Review.
-
A role for the vitamin D pathway in non-intestinal lesions in genetic and carcinogen models of colorectal cancer and in familial adenomatous polyposis.Oncotarget. 2016 Dec 6;7(49):80508-80520. doi: 10.18632/oncotarget.12768. Oncotarget. 2016. PMID: 27768599 Free PMC article.
-
Vitamin D and VDR gene polymorphism (FokI) in epithelial ovarian cancer in Indian population.J Ovarian Res. 2013 May 26;6(1):37. doi: 10.1186/1757-2215-6-37. J Ovarian Res. 2013. PMID: 23705897 Free PMC article.
-
Current evidence for vitamin D in intestinal function and disease.Exp Biol Med (Maywood). 2019 Sep;244(12):1040-1052. doi: 10.1177/1535370219867262. Epub 2019 Jul 31. Exp Biol Med (Maywood). 2019. PMID: 31366237 Free PMC article. Review.
References
-
- Wood RJ, Fleet JC. The genetics of osteoporosis: vitamin D receptor polymorphisms. Annu Rev Nutr. 1998;18:233–258. - PubMed
-
- Jurutka PW, Remus LS, Whitfield GK, et al. The polymorphic N terminus in human vitamin D receptor isoforms influences transcriptional activity by modulating interaction with transcription factor IIB. Mol Endocrinol. 2000;14(3):401–420. - PubMed
-
- Park K, Woo M, Nam J, Kim JC. Start codon polymorphisms in the vitamin D receptor and colorectal cancer risk. Cancer Lett. 2006;237(2):199–206. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

