Identification of furan metabolites derived from cysteine-cis-2-butene-1,4-dial-lysine cross-links
- PMID: 20043645
- PMCID: PMC2826838
- DOI: 10.1021/tx9003215
Identification of furan metabolites derived from cysteine-cis-2-butene-1,4-dial-lysine cross-links
Abstract
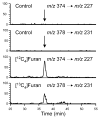
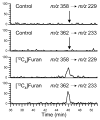
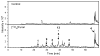
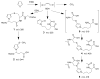
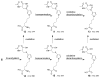
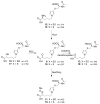
Furan is a rodent hepatotoxicant and carcinogen. Because this compound is an important industrial intermediate and has been detected in heat-processed foods and smoke, humans are likely exposed to this toxic compound. Characterization of urinary metabolites of furan will lead to the development of biomarkers to assess human health risks associated with furan exposure. Previous studies indicate that furan is oxidized to a reactive alpha,beta-unsaturated dialdehyde, cis-2-butene-1,4-dial (BDA), in a reaction catalyzed by cytochrome P450. Five previously characterized metabolites are derived from the reaction of BDA with cellular nucleophiles such as glutathione and protein. They include the monoglutathione reaction product, N-[4-carboxy-4-(3-mercapto-1H-pyrrol-1-yl)-1-oxobutyl]-l-cysteinylglycine cyclic sulfide, and its downstream metabolite, S-[1-(1,3-dicarboxypropyl)-1H-pyrrol-3-yl]methylthiol, as well as (R)-2-acetylamino-6-(2,5-dihydro-2-oxo-1H-pyrrol-1-yl)-1-hexanoic acid and N-acetyl-S-[1-(5-acetylamino-5-carboxypentyl)-1H-pyrrol-3-yl]-l-cysteine and its sulfoxide. The last two compounds are downstream metabolites of a BDA-derived cysteine-lysine cross-link, S-[1-(5-amino-5-carboxypentyl)-1H-pyrrol-3-yl]-l-cysteine. In this report, we present the characterization of seven additional urinary furan metabolites, all of which are derived from this cross-link. The cysteinyl residue is subject to several biotransformation reactions, including N-acetylation and S-oxidation. Alternatively, it can undergo beta-elimination followed by S-methylation to a methylthiol intermediate that is further oxidized to a sulfoxide. The lysine portion of the cross-link either is N-acetylated or undergoes a transamination reaction to generate an alpha-ketoacid metabolite that undergoes oxidative decarboxylation. Some of these metabolites are among the most abundant furan metabolites present in urine as judged by LC-MS/MS analysis, indicating that the oxidation of furan to BDA and BDA's subsequent reaction with cellular cysteine and lysine residues may represent a significant in vivo pathway of furan biotransformation. Because they are derived from cellular BDA reaction products, these metabolites are markers of furan exposure and bioactivation and could be explored as potential biomarkers in human studies.
Figures


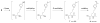
Similar articles
-
Degraded protein adducts of cis-2-butene-1,4-dial are urinary and hepatocyte metabolites of furan.Chem Res Toxicol. 2009 Jun;22(6):997-1007. doi: 10.1021/tx800377v. Chem Res Toxicol. 2009. PMID: 19441776 Free PMC article.
-
Validation of putative biomarkers of furan exposure through quantitative analysis of furan metabolites in urine of F344 rats exposed to stable isotope labeled furan.Arch Toxicol. 2024 Jun;98(6):1741-1756. doi: 10.1007/s00204-024-03722-5. Epub 2024 Apr 4. Arch Toxicol. 2024. PMID: 38573339 Free PMC article.
-
Trapping of cis-2-butene-1,4-dial to measure furan metabolism in human liver microsomes by cytochrome P450 enzymes.Drug Metab Dispos. 2012 Mar;40(3):596-601. doi: 10.1124/dmd.111.043679. Epub 2011 Dec 20. Drug Metab Dispos. 2012. PMID: 22187484 Free PMC article.
-
Electrophilic intermediates produced by bioactivation of furan.Drug Metab Rev. 2006;38(4):615-26. doi: 10.1080/03602530600959417. Drug Metab Rev. 2006. PMID: 17145691 Review.
-
Reactive metabolites in the biotransformation of molecules containing a furan ring.Chem Res Toxicol. 2013 Jan 18;26(1):6-25. doi: 10.1021/tx3003824. Epub 2012 Oct 24. Chem Res Toxicol. 2013. PMID: 23061605 Free PMC article. Review.
Cited by
-
Abundant Rodent Furan-Derived Urinary Metabolites Are Associated with Tobacco Smoke Exposure in Humans.Chem Res Toxicol. 2015 Jul 20;28(7):1508-16. doi: 10.1021/acs.chemrestox.5b00189. Epub 2015 Jul 7. Chem Res Toxicol. 2015. PMID: 26114498 Free PMC article.
-
Biomarker-based approach to human exposure assessment of furan in food.Arch Toxicol. 2025 Jul;99(7):2819-2834. doi: 10.1007/s00204-025-04022-2. Epub 2025 Apr 4. Arch Toxicol. 2025. PMID: 40183809 Free PMC article.
-
Inhaled Furan Selectively Damages Club Cells in Lungs of A/J Mice.Toxicol Pathol. 2019 Oct;47(7):842-850. doi: 10.1177/0192623319869306. Epub 2019 Aug 19. Toxicol Pathol. 2019. PMID: 31426723 Free PMC article.
-
Stable Isotope Dilution Analysis (SIDA) to Determine Metabolites of Furan and 2-Methylfuran in Human Urine Samples: A Pilot Study.Metabolites. 2023 Sep 14;13(9):1011. doi: 10.3390/metabo13091011. Metabolites. 2023. PMID: 37755292 Free PMC article.
-
Chemical Identity of Interaction of Protein with Reactive Metabolite of Diosbulbin B In Vitro and In Vivo.Toxins (Basel). 2017 Aug 14;9(8):249. doi: 10.3390/toxins9080249. Toxins (Basel). 2017. PMID: 28805726 Free PMC article.
References
-
- International Agency for Research on Cancer. Dry Cleaning, Some Chlorinated Solvents and Other Industrial Chemicals. IARC; Lyon, France: 1995. Furan; p. 393.
-
- Crews C, Castle L. A review of the occurrence, formation and analysis of furan in heat-processed foods. Trends Food Sci Technol. 2007;18:365–372.
-
- National Toxicology Program. Toxicology and carcinogenesis studies of benzofuran in F344/N rats and B6C3F1 mice vol. US Department of Health and Human Services, Public Health Service, National Institutes of Health; Research Triangle Park, NC: 1989. NTP Technical Report No. 370. - PubMed
-
- Burka LT, Washburn KD, Irwin RD. Disposition of [14C]furan in the male F344 rat. J Toxicol Environ Health. 1991;34:245–257. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

