Myeloperoxidase: an oxidative pathway for generating dysfunctional high-density lipoprotein
- PMID: 20043647
- PMCID: PMC2838938
- DOI: 10.1021/tx9003775
Myeloperoxidase: an oxidative pathway for generating dysfunctional high-density lipoprotein
Abstract
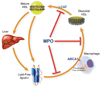
Accumulation of low-density lipoprotein (LDL)-derived cholesterol by artery wall macrophages triggers atherosclerosis, the leading cause of cardiovascular disease. Conversely, high-density lipoprotein (HDL) retards atherosclerosis by promoting cholesterol efflux from macrophages by the membrane-associated ATP-binding cassette transporter A1 (ABCA1) pathway. HDL has been proposed to lose its cardioprotective effects in subjects with atherosclerosis, but the underlying mechanisms are poorly understood. One potential pathway involves oxidative damage by myeloperoxidase (MPO), a heme enzyme secreted by human artery wall macrophages. We used mass spectrometry to demonstrate that HDL isolated from patients with established cardiovascular disease contains elevated levels of 3-chlorotyrosine and 3-nitrotyrosine, two characteristic products of MPO. When apolipoprotein A-I (apoA-I), the major HDL protein, was oxidized by MPO, its ability to promote cellular cholesterol efflux by ABCA1 was impaired. Moreover, oxidized apoA-I was unable to activate lecithin:cholesterol acyltransferase (LCAT), which rapidly converts free cholesterol to cholesteryl ester, a critical step in HDL maturation. Biochemical studies implicated tyrosine chlorination and methionine oxygenation in the loss of ABCA1 and LCAT activity by oxidized apoA-I. Oxidation of specific residues in apoA-I inhibited two key steps in cholesterol efflux from macrophages, raising the possibility that MPO initiates a pathway for generating dysfunctional HDL in humans.
Figures





Similar articles
-
Site-specific oxidation of apolipoprotein A-I impairs cholesterol export by ABCA1, a key cardioprotective function of HDL.Biochim Biophys Acta. 2012 Mar;1821(3):490-501. doi: 10.1016/j.bbalip.2011.11.011. Epub 2011 Dec 10. Biochim Biophys Acta. 2012. PMID: 22178192 Free PMC article. Review.
-
Pathways for oxidation of high-density lipoprotein in human cardiovascular disease.Curr Opin Mol Ther. 2006 Jun;8(3):198-205. Curr Opin Mol Ther. 2006. PMID: 16774039 Review.
-
Humans with atherosclerosis have impaired ABCA1 cholesterol efflux and enhanced high-density lipoprotein oxidation by myeloperoxidase.Circ Res. 2014 May 23;114(11):1733-42. doi: 10.1161/CIRCRESAHA.114.303454. Epub 2014 Mar 19. Circ Res. 2014. PMID: 24647144 Free PMC article.
-
Myeloperoxidase Targets Apolipoprotein A-I for Site-Specific Tyrosine Chlorination in Atherosclerotic Lesions and Generates Dysfunctional High-Density Lipoprotein.Chem Res Toxicol. 2021 Jun 21;34(6):1672-1680. doi: 10.1021/acs.chemrestox.1c00086. Epub 2021 Apr 16. Chem Res Toxicol. 2021. PMID: 33861588
-
Impact of HDL oxidation by the myeloperoxidase system on sterol efflux by the ABCA1 pathway.J Proteomics. 2011 Oct 19;74(11):2289-99. doi: 10.1016/j.jprot.2011.04.001. Epub 2011 Apr 9. J Proteomics. 2011. PMID: 21501700 Free PMC article.
Cited by
-
The Enzymatic and Non-Enzymatic Function of Myeloperoxidase (MPO) in Inflammatory Communication.Antioxidants (Basel). 2021 Apr 5;10(4):562. doi: 10.3390/antiox10040562. Antioxidants (Basel). 2021. PMID: 33916434 Free PMC article. Review.
-
Rapid degradation of ABCA1 protein following cAMP withdrawal and treatment with PKA inhibitor suggests ABCA1 is a short-lived protein primarily regulated at the transcriptional level.J Diabetes Metab Disord. 2020 Mar 19;19(1):363-371. doi: 10.1007/s40200-020-00517-0. eCollection 2020 Jun. J Diabetes Metab Disord. 2020. PMID: 32550187 Free PMC article.
-
HDL from apoA1 transgenic mice expressing the 4WF isoform is resistant to oxidative loss of function.J Lipid Res. 2015 Mar;56(3):653-664. doi: 10.1194/jlr.M056754. Epub 2015 Jan 5. J Lipid Res. 2015. PMID: 25561462 Free PMC article.
-
Sequence conservation of apolipoprotein A-I affords novel insights into HDL structure-function.J Lipid Res. 2011 Mar;52(3):435-50. doi: 10.1194/jlr.R012658. Epub 2010 Dec 14. J Lipid Res. 2011. PMID: 21159667 Free PMC article. Review.
-
Free Radicals and Obesity-Related Chronic Inflammation Contrasted by Antioxidants: A New Perspective in Coronary Artery Disease.Metabolites. 2023 May 31;13(6):712. doi: 10.3390/metabo13060712. Metabolites. 2023. PMID: 37367870 Free PMC article. Review.
References
-
- Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. - PubMed
-
- Brown MS, Goldstein JL. Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu Rev Biochem. 1983;52:223–261. - PubMed
-
- Berliner JA, Heinecke JW. The role of oxidized lipoproteins in atherogenesis. Free Radic Biol Med. 1996;20:707–727. - PubMed
-
- Stadtman ER. Protein oxidation and aging. Science. 1992;257:1220–1224. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

