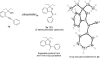A simple and mild synthesis of 1H-isochromenes and (Z)-1-alkylidene-1,3-dihydroisobenzofurans by the iodocyclization of 2-(1-alkynyl)benzylic alcohols
- PMID: 20043652
- PMCID: PMC2813916
- DOI: 10.1021/jo902333y
A simple and mild synthesis of 1H-isochromenes and (Z)-1-alkylidene-1,3-dihydroisobenzofurans by the iodocyclization of 2-(1-alkynyl)benzylic alcohols
Abstract
A variety of iodo-substituted isochromenes, dihydroisobenzofurans, and pyranopyridines are readily prepared in good to excellent yields under mild conditions by the iodocyclization of readily available 2-(1-alkynyl)benzylic alcohols or 2-(1-alkynyl)-3-(hydroxymethyl)pyridines. Reactions are carried out in MeCN at 25 degrees C with 3 equiv of I(2) as the iodine source and NaHCO(3) (3 equiv) as the base. The regiochemical outcome of the reaction strongly depends on the substitution pattern of the starting material. In particular, the 5-exo-dig cyclization mode, leading to dihydroisobenzofurans, is observed in the case of substrates bearing a tertiary alcoholic group, owing to the gem-dialkyl effect, while the 6-endo-dig cyclization mode, leading to isochromene or pyranopyridines, is the usually preferred pathway in the case of substrates bearing a primary or secondary alcoholic group.
Figures
References
-
- Arcadi A, Cacchi S, Fabrizi G, Marinelli F, Moro L. Synlett. 1999:1432.
- Yue D, Yao T, Larock RC. J. Org. Chem. 2005;70:10292. - PMC - PubMed
- Yue D, Yao T, Larock RC. J. Comb. Chem. 2005;7:809. - PubMed
- Manarin F, Roehrs JA, Gay RM, Brandão R, Menezes PH, Nogueira CW, Zeni G. J. Org. Chem. 2009;74:2153. - PubMed
-
- Sniady A, Wheeler KA, Dembinski R. Org. Lett. 2005;7:1769. - PubMed
- Yao T, Zhang X, Larock RC. J. Org. Chem. 2005;70:7679. - PubMed
- Liu Y, Zhou S. Org. Lett. 2005;7:4609. - PubMed
- Yao T, Zhang X, Larock RC. J. Am. Chem. Soc. 2004;126:11164. - PubMed
- Bew SP, El-Taeb GMM, Jones S, Knight DW, Tan W. Eur. J. Org. Chem. 2007:5759.
- Arimitsu S, Jacobsen JM, Hammond GB. J. Org. Chem. 2008;73:2886. - PubMed
- Huang X, Fu W, Miao M. Tetrahedron Lett. 2008;49:2359.
-
- Flynn BL, Flynn GP, Hamel E, Jung MK. Bioorg. Med. Chem. Lett. 2001;11:2341. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials