Cost-effectiveness of tenofovir as first-line antiretroviral therapy in India
- PMID: 20043752
- PMCID: PMC3225050
- DOI: 10.1086/649884
Cost-effectiveness of tenofovir as first-line antiretroviral therapy in India
Abstract
Background: World Health Organization guidelines for antiretroviral treatment (ART) in resource-limited settings recommend either stavudine or tenofovir as part of initial therapy. We evaluated the clinical outcomes and cost-effectiveness of first-line ART using tenofovir in India, compared with current practice using stavudine or zidovudine.
Methods: We used a state-transition model of human immunodeficiency virus (HIV) disease to examine strategies using different nucleoside reverse-transcriptase inhibitors, combined with lamivudine and nevirapine, compared with no ART: (1) stavudine, (2) stavudine with substitution by zidovudine after 6 months, (3) zidovudine, and (4) tenofovir. Data were from the Y. R. Gaitonde Centre for AIDS Research and Education in Chennai, India, and published studies. Results. Discounted mean per person survival was 36.9 months (40.2 months undiscounted) with no ART, 115.5 months (145.3) with stavudine-containing ART, 115.7 months (145.6) with stavudine and 6-month zidovudine substitution, 115.8 months (145.6) with zidovudine-containing ART, and 125.8 months (162.0) with initial tenofovir. Discounted lifetime medical costs were $610 with no ART and ranged from $5580 with stavudine-containing ART to $5720 with zidovudine-containing ART. Initial tenofovir had an incremental cost-effectiveness ratio of $670 per year of life saved, compared with no ART, and was more economically efficient than the other regimens.
Results: were most sensitive to variations in the costs of first-line tenofovir, access to additional ART after treatment failure, and quality of life adjustment.
Conclusions: Using tenofovir as part of first-line ART in India will improve survival, is cost-effective by international standards, and should be considered for initial therapy for HIV-infected patients in India.
Conflict of interest statement
Figures
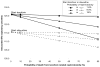
 or
or
 ), 8.0% (
), 8.0% (
 or
or
 ), and 16.0% (
), and 16.0% (
 or
or
 ).
).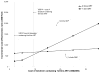
 ), and for the scenario in which only one line of ART is available (
), and for the scenario in which only one line of ART is available (
 ). For the two-line scenario, the costs of the tenofovir component of second-line ART were assumed to rise commensurate with first-line cost increases; thus, the cost of tenofovir-containing second-line ART was varied from $49-$142 (not shown). These cost-effectiveness results are reported compared to no ART. For the one-line scenario, as the cost of initial tenofovir increases, the incremental cost-effectiveness ratios are reported compared to no ART (for initial tenofovir costs of $7 and $10), and to initial stavudine-to-zidovudine (for costs greater than $10). ART: antiretroviral therapy; YLS: year of life saved; GDP: gross domestic product.
). For the two-line scenario, the costs of the tenofovir component of second-line ART were assumed to rise commensurate with first-line cost increases; thus, the cost of tenofovir-containing second-line ART was varied from $49-$142 (not shown). These cost-effectiveness results are reported compared to no ART. For the one-line scenario, as the cost of initial tenofovir increases, the incremental cost-effectiveness ratios are reported compared to no ART (for initial tenofovir costs of $7 and $10), and to initial stavudine-to-zidovudine (for costs greater than $10). ART: antiretroviral therapy; YLS: year of life saved; GDP: gross domestic product.References
-
- UNAIDS. [Accessed 13 March 2009];2.5 million people in India living with HIV, according to new estimates. 2007 Available at: http://data.unaids.org/pub/PressRelease/2007/070706_indiapressrelease_en....
-
- Department of Health and Human Services (DHHS) Panel on Antiretroviral Guidelines for Adults and Adolescents. [Accessed 13 March 2009];Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2008 Available at: http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL.pdf.
-
- World Health Organization (WHO) [Accessed 13 March 2009];Antiretroviral therapy for HIV infection in adults and adolescents in resource-limited settings: Towards universal access. 2006 Available at: http://www.who.int/hiv/pub/guidelines/adult/en/index.html.
-
- The Clinton Foundation. [Accessed 13 March 2009];Antiretroviral (ARV) price list. 2008 Available at: http://www.clintonfoundation.org/download/?guid=62e82ddc-98de-102b-be34-....
-
- Kumarasamy N, Venkatesh KK, Cecelia AJ, et al. Spectrum of adverse events after generic HAART in southern Indian HIV-infected patients. AIDS Patient Care STDS. 2008 Apr;22(4):337–44. - PubMed
APPENDIX REFERENCES
-
- Hammer SM, Squires KE, Hughes MD, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med. 1997 Sep 11;337(11):725–33. - PubMed
-
- Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998 Mar 26;338(13):853–60. - PubMed
-
- Mellors JW, Munoz A, Giorgi JV, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997 Jun 15;126(12):946–54. - PubMed
-
- Multicenter AIDS Cohort Study (MACS) Public Dataset: Release PO4. National Technical Information Service; 1995.
-
- Wang B, Kumarasamy N, Divi N, et al. Incidence of opportunistic infections (OIs) within specific CD4 strata in HIV-infected patients in Southern India [abstract WePe0279]. XVI International AIDS Conference; August 16, 2006; Toronto, Canada.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

