Conservation of intron and intein insertion sites: implications for life histories of parasitic genetic elements
- PMID: 20043855
- PMCID: PMC2814812
- DOI: 10.1186/1471-2148-9-303
Conservation of intron and intein insertion sites: implications for life histories of parasitic genetic elements
Abstract
Background: Inteins and introns are genetic elements that are removed from proteins and RNA after translation or transcription, respectively. Previous studies have suggested that these genetic elements are found in conserved parts of the host protein. To our knowledge this type of analysis has not been done for group II introns residing within a gene. Here we provide quantitative statistical support from an analyses of proteins that host inteins, group I introns, group II introns and spliceosomal introns across all three domains of life.
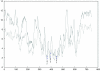
Results: To determine whether or not inteins, group I, group II, and spliceosomal introns are found preferentially in conserved regions of their respective host protein, conservation profiles were generated and intein and intron positions were mapped to the profiles. Fisher's combined probability test was used to determine the significance of the distribution of insertion sites across the conservation profile for each protein. For a subset of studied proteins, the conservation profile and insertion positions were mapped to protein structures to determine if the insertion sites correlate to regions of functional activity. All inteins and most group I introns were found to be preferentially located within conserved regions; in contrast, a bacterial intein-like protein, group II and spliceosomal introns did not show a preference for conserved sites.
Conclusions: These findings demonstrate that inteins and group I introns are found preferentially in conserved regions of their respective host proteins. Homing endonucleases are often located within inteins and group I introns and these may facilitate mobility to conserved regions. Insertion at these conserved positions decreases the chance of elimination, and slows deletion of the elements, since removal of the elements has to be precise as not to disrupt the function of the protein. Furthermore, functional constrains on the targeted site make it more difficult for hosts to evolve immunity to the homing endonuclease. Therefore, these elements will better survive and propagate as molecular parasites in conserved sites. In contrast, spliceosomal introns and group II introns do not show significant preference for conserved sites and appear to have adopted a different strategy to evade loss.
Figures
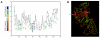



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

