Lipotoxicity-mediated cell dysfunction and death involve lysosomal membrane permeabilization and cathepsin L activity
- PMID: 20043885
- PMCID: PMC2829674
- DOI: 10.1016/j.brainres.2009.12.038
Lipotoxicity-mediated cell dysfunction and death involve lysosomal membrane permeabilization and cathepsin L activity
Abstract
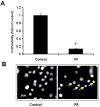
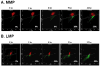
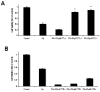
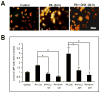
Lipotoxicity, which is triggered when cells are exposed to elevated levels of free fatty acids, involves cell dysfunction and apoptosis and is emerging as an underlying factor contributing to various pathological conditions including disorders of the central nervous system and diabetes. We have shown that palmitic acid (PA)-induced lipotoxicity (PA-LTx) in nerve growth factor-differentiated PC12 (NGFDPC12) cells is linked to an augmented state of cellular oxidative stress (ASCOS) and apoptosis and that these events are inhibited by docosahexanoic acid (DHA). The mechanisms of PA-LTx in nerve cells are not well understood, but our previous findings indicate that it involves ROS generation, mitochondrial membrane permeabilization (MMP), and caspase activation. The present study used nerve growth factor differentiated PC12 cells (NGFDPC12 cells) and found that lysosomal membrane permeabilization (LMP) is an early event during PA-induced lipotoxicity that precedes MMP and apoptosis. Cathepsin L, but not cathepsin B, is an important contributor in this process since its pharmacological inhibition significantly attenuated LMP, MMP, and apoptosis. In addition, co-treatment of NGFDPC12 cells undergoing lipotoxicity with DHA significantly reduced LMP, suggesting that DHA acts by antagonizing upstream signals leading to lysosomal dysfunction. These results suggest that LMP is a key early mediator of lipotoxicity and underscore the value of interventions targeting upstream signals leading to LMP for the treatment of pathological conditions associated with lipotoxicity.
Copyright 2009 Elsevier B.V. All rights reserved.
Figures


References
-
- Arab K, Rossary A, Flourié F, Tourneur Y, Steghens JP. Docosahexaenoic acid enhances the antioxidant response of human fibroblasts by upregulating gamma-glutamyl-cysteinyl ligase and glutathione reductase. Br J Nutr. 2006;95:18–26. - PubMed
-
- Bas O, Songur A, Sahin O, Mollaoglu H, Ozen OA, Yaman M, Eser O, Fidan H, Yagmurca M. The protective effect of fish n-3 fatty acids on cerebral ischemia in rat hippocampus. Neurochem Int. 2007;50:548–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

