Threshold size for optimal passive pulmonary targeting and retention of rigid microparticles in rats
- PMID: 20043961
- PMCID: PMC2840186
- DOI: 10.1016/j.jconrel.2009.12.019
Threshold size for optimal passive pulmonary targeting and retention of rigid microparticles in rats
Abstract
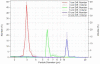
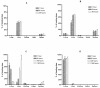
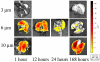
The relationship between microparticle (MP) size and lung targeting efficiency, intra-lung distribution and retention time was systematically studied after intravenous administration of rigid fluorescent polystyrene MPs of various sizes (2, 3, 6 and 10 microm) to Sprague Dawley rats. Total fluorescence was assessed and it was found that 2 microm and 3 microm MPs readily passed through the lung to the liver and spleen while 10 microm MPs were completely entrapped in the lung for the one-week duration of the study. Approximately 84% of 6 microm MPs that were initially entrapped in the lung were cleared over the next 2 days and 15% were cleared over the remaining 5 days. A Caliper IVIS 100 small animal imaging system confirmed that 3 microm MPs were not retained in the lung but that 6 microm and 10 microm MPs were widely distributed throughout the lung. Moreover, histologic examination showed MP entrapment in capillaries but not arterioles. These studies suggest that for rigid MPs the optimal size range required to achieve transient but highly efficiently targeting to pulmonary capillaries after IV injection is >6 microm but <10 microm in rats and that systemic administration of optimally sized MPs may be an efficient alternative to currently used inhalation-based delivery to the lung.
Copyright 2009 Elsevier B.V. All rights reserved.
Figures


References
-
- Pritchard JN. The influence of lung deposition on clinical response. J Aerosol Med. 2001;14(Suppl 1):S19–26. - PubMed
-
- Derendorf H, Hochhaus G, Mollmann H. Evaluation of pulmonary absorption using pharmacokinetic methods. J Aerosol Med. 2001;14(Suppl 1):S9–17. - PubMed
-
- Washington N, Washington C, Wilson CG. Physiological pharmaceutics: barriers to drug absorption. Taylor & Francis; New York: 2001. pp. 221–247.
-
- Newman SP, Steed KP, Hooper G, Jones JI, Upchurch FC. Improved targeting of beclomethasone diproprionate (250 micrograms metered dose inhaler) to the lungs of asthmatics with the Spacehaler. Respir Med. 1999;93(6):424–431. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

