Regulation of phagocyte NADPH oxidase by hydrogen peroxide through a Ca(2+)/c-Abl signaling pathway
- PMID: 20043988
- PMCID: PMC2838729
- DOI: 10.1016/j.freeradbiomed.2009.12.018
Regulation of phagocyte NADPH oxidase by hydrogen peroxide through a Ca(2+)/c-Abl signaling pathway
Abstract
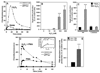
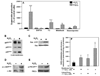
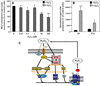
The importance of H(2)O(2) as a cellular signaling molecule has been demonstrated in a number of cell types and pathways. Here we explore a positive feedback mechanism of H(2)O(2)-mediated regulation of the phagocyte respiratory burst NADPH oxidase (NOX2). H(2)O(2) induced a dose-dependent stimulation of superoxide production in human neutrophils, as well as in K562 leukemia cells overexpressing NOX2 system components. Stimulation was abrogated by the addition of catalase, the extracellular Ca(2+) chelator BAPTA, the T-type Ca(2+) channel inhibitor mibefradil, the PKCdelta inhibitor rottlerin, or the c-Abl nonreceptor tyrosine kinase inhibitor imatinib mesylate or by overexpression of a dominant-negative form of c-Abl. H(2)O(2) induced phosphorylation of tyrosine 311 on PKCdelta and this activating phosphorylation was blocked by treatment with rottlerin, imatinib mesylate, or BAPTA. Rac GTPase activation in response to H(2)O(2) was abrogated by BAPTA, imatinib mesylate, or rottlerin. In conclusion, H(2)O(2) stimulates NOX2-mediated superoxide generation in neutrophils and K562/NOX2 cells via a signaling pathway involving Ca(2+) influx and c-Abl tyrosine kinase acting upstream of PKCdelta. This positive feedback regulatory pathway has important implications for amplifying the innate immune response and contributing to oxidative stress in inflammatory disorders.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures






Similar articles
-
Novel redox-dependent regulation of NOX5 by the tyrosine kinase c-Abl.Free Radic Biol Med. 2008 Mar 1;44(5):868-81. doi: 10.1016/j.freeradbiomed.2007.11.020. Epub 2007 Dec 8. Free Radic Biol Med. 2008. PMID: 18160052 Free PMC article.
-
Characterization of superoxide overproduction by the D-Loop(Nox4)-Nox2 cytochrome b(558) in phagocytes-Differential sensitivity to calcium and phosphorylation events.Biochim Biophys Acta. 2011 Jan;1808(1):78-90. doi: 10.1016/j.bbamem.2010.08.002. Epub 2010 Aug 11. Biochim Biophys Acta. 2011. PMID: 20708598 Free PMC article.
-
Arachidonic acid induces direct interaction of the p67(phox)-Rac complex with the phagocyte oxidase Nox2, leading to superoxide production.J Biol Chem. 2014 Sep 5;289(36):24874-84. doi: 10.1074/jbc.M114.581785. Epub 2014 Jul 23. J Biol Chem. 2014. PMID: 25056956 Free PMC article.
-
p47phox, the phagocyte NADPH oxidase/NOX2 organizer: structure, phosphorylation and implication in diseases.Exp Mol Med. 2009 Apr 30;41(4):217-25. doi: 10.3858/emm.2009.41.4.058. Exp Mol Med. 2009. PMID: 19372727 Free PMC article. Review.
-
Do Hv1 proton channels regulate the ionic and redox homeostasis of phagosomes?Mol Cell Endocrinol. 2012 Apr 28;353(1-2):82-7. doi: 10.1016/j.mce.2011.10.005. Epub 2011 Oct 26. Mol Cell Endocrinol. 2012. PMID: 22056415 Review.
Cited by
-
Role of H(2)O(2) in hypertension, renin-angiotensin system activation and renal medullary disfunction caused by angiotensin II.Br J Pharmacol. 2012 Aug;166(8):2386-401. doi: 10.1111/j.1476-5381.2012.01957.x. Br J Pharmacol. 2012. PMID: 22452317 Free PMC article.
-
Pyocyanin-enhanced neutrophil extracellular trap formation requires the NADPH oxidase.PLoS One. 2013;8(1):e54205. doi: 10.1371/journal.pone.0054205. Epub 2013 Jan 14. PLoS One. 2013. PMID: 23342104 Free PMC article.
-
Characterization of Virulence Factors of Staphylococcus aureus: Novel Function of Known Virulence Factors That Are Implicated in Activation of Airway Epithelial Proinflammatory Response.J Pathog. 2011;2011:601905. doi: 10.4061/2011/601905. Epub 2011 Sep 14. J Pathog. 2011. PMID: 22567334 Free PMC article.
-
Reactive Oxygen Species in Venous Thrombosis.Int J Mol Sci. 2020 Mar 11;21(6):1918. doi: 10.3390/ijms21061918. Int J Mol Sci. 2020. PMID: 32168908 Free PMC article. Review.
-
PRBC-derived plasma induces non-muscle myosin type IIA-mediated neutrophil migration and morphologic change.Immunopharmacol Immunotoxicol. 2013 Feb;35(1):71-9. doi: 10.3109/08923973.2012.677046. Epub 2012 Oct 19. Immunopharmacol Immunotoxicol. 2013. PMID: 23083320 Free PMC article.
References
-
- Bedard K, Krause K-H. The NOX Family of ROS-Generating NADPH Oxidases: Physiology and Pathophysiology. Physiol. Rev. 2007;87:245–313. - PubMed
-
- Han CH, Freeman JL, Lee T, Motalebi SA, Lambeth JD. Regulation of the neutrophil respiratory burst oxidase. Identification of an activation domain in p67(phox) J. Biol. Chem. 1998;273:16663–16668. - PubMed
-
- Bokoch GM, Ulla GK. NADPH oxidases: not just for leukocytes anymore! Trends Biochem. Sci. 2003;28:502–508. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

