Agonist-dependent mu-opioid receptor signaling can lead to heterologous desensitization
- PMID: 20043990
- PMCID: PMC2833358
- DOI: 10.1016/j.cellsig.2009.12.003
Agonist-dependent mu-opioid receptor signaling can lead to heterologous desensitization
Abstract
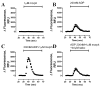
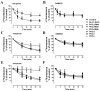
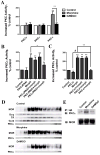
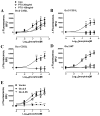
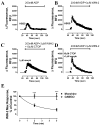
Desensitization of the micro-opioid receptor (MOR) has been implicated as an important regulatory process in the development of tolerance to opiates. Monitoring the release of intracellular Ca(2+) ([Ca(2+)](i)), we reported that [D-Ala(2), N-Me-Phe(4), Gly(5)-ol]-enkephalin (DAMGO)-induced receptor desensitization requires receptor phosphorylation and recruitment of beta-arrestins (betaArrs), while morphine-induced receptor desensitization does not. In current studies, we established that morphine-induced MOR desensitization is protein kinase C (PKC)-dependent. By using RNA interference techniques and subtype specific inhibitors, PKCepsilon was shown to be the PKC subtype activated by morphine and the subtype responsible for morphine-induced desensitization. In contrast, DAMGO did not increase PKCepsilon activity and DAMGO-induced MOR desensitization was not affected by modulating PKCepsilon activity. Among the various proteins within the receptor signaling complex, Galphai2 was phosphorylated by morphine-activated PKCepsilon. Moreover, mutating three putative PKC phosphorylation sites, Ser(44), Ser(144) and Ser(302) on Galphai2 to Ala attenuated morphine-induced, but not DAMGO-induced desensitization. In addition, pretreatment with morphine desensitized cannabinoid receptor CB1 agonist WIN 55212-2-induced [Ca(2+)](i) release, and this desensitization could be reversed by pretreating the cells with PKCepsilon inhibitor or overexpressing Galphai2 with the putative PKC phosphorylation sites mutated. Thus, depending on the agonist, activation of MOR could lead to heterologous desensitization and probable crosstalk between MOR and other Galphai-coupled receptors, such as the CB1.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures



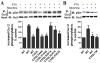
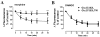

References
-
- Borgland SL. Clin Exp Pharmacol Physiol. 2001;28(3):147–154. - PubMed
-
- Lefkowitz RJ. J Biol Chem. 1998;273(30):18677–18680. - PubMed
-
- Violin JD, Lefkowitz RJ. Trends Pharmacol Sci. 2007;28(8):416–422. - PubMed
-
- Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG. Annu Rev Neurosci. 2004;27:107–144. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DA007339/DA/NIDA NIH HHS/United States
- R01 DA016674/DA/NIDA NIH HHS/United States
- K05 DA000513/DA/NIDA NIH HHS/United States
- R56 DA000564/DA/NIDA NIH HHS/United States
- K05-DA00513/DA/NIDA NIH HHS/United States
- K05 DA070554/DA/NIDA NIH HHS/United States
- DA016674/DA/NIDA NIH HHS/United States
- DA011806/DA/NIDA NIH HHS/United States
- DA000564/DA/NIDA NIH HHS/United States
- R01 DA000564/DA/NIDA NIH HHS/United States
- K05-DA70544/DA/NIDA NIH HHS/United States
- P50 DA011806/DA/NIDA NIH HHS/United States
- R01 DA007339/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

