Structural insights into interferon regulatory factor activation
- PMID: 20043992
- PMCID: PMC2846214
- DOI: 10.1016/j.cellsig.2009.12.005
Structural insights into interferon regulatory factor activation
Abstract
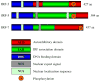
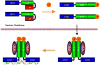
The interferon regulatory factors (IRFs) play important roles in development of the immune system and host defense. Recent crystallographic and biochemical studies have provided insights into the mechanism of activation of IRFs by phosphorylation. The activation of a latent closed conformation of IRF in the cytoplasm is triggered by phosphorylation of Ser/Thr residues in a C-terminal region. Phosphorylation stimulates the C-terminal autoinhibitory domain to attain a highly extended conformation triggering dimerization through extensive contacts to a second subunit. Dimers are then transported into the nucleus and assemble with the coactivator CBP/p300 to activate transcription of type I interferons and other target genes. The advances made in understanding the release of inhibition after IRF dimerization have generated a detailed structural model of how IRFs signaling pathways are activated.
Published by Elsevier Inc.
Figures

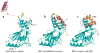
References
-
- Tamura T, Yanai H, Savitsky D, Taniguchi T. Annu Rev Immunol. 2008;26:535–584. - PubMed
-
- Hu G, Mancl ME, Barnes BJ. Cancer Res. 2005;65(16):7403–7412. - PubMed
-
- Alter-Koltunoff M, Goren S, Nousbeck J, Feng CG, Sher A, Ozato K, Azriel A, Levi BZ. J Biol Chem. 2008;283(5):2724–2733. - PubMed
-
- Ozato K, Tailor P, Kubota T. J Biol Chem. 2007;282(28):20065–20069. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

