Modulation of feeding and locomotion through mu and delta opioid receptor signaling in the nucleus accumbens
- PMID: 20044138
- PMCID: PMC2854292
- DOI: 10.1016/j.npep.2009.12.002
Modulation of feeding and locomotion through mu and delta opioid receptor signaling in the nucleus accumbens
Abstract
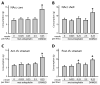
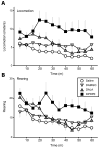
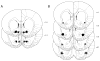
Opioid signaling has been strongly implicated in driving palatable food consumption. The nucleus accumbens (NAcc) is one important site of this effect; hyperphagia elicited by administration of exogenous mu opioid receptor (MOR) ligands in this brain region has been well documented. However, the role that endogenous opioid ligands in the NAcc play in controlling food intake remains poorly understood. Enkephalins, which signal through both the MOR and delta opioid receptor (DOR), are highly expressed within a subset of NAcc neurons, and have been shown to be sensitive to manipulations of diet and motivation. To investigate a potential role for these signaling molecules in regulating palatability-driven consumption, we measured high fat chow intake in rats following a series of pharmacological manipulations of NAcc opioid signaling. NAcc infusion of the MOR agonist [D-Ala2, N-MePHe4, Gly-ol]-enkephalin (DAMGO) robustly increased palatable food intake, as has previously been demonstrated. In contrast, neither infusion of Met-enkephalin, its synthetic analogue [D-Ala2] Met-enkephalin (DALA) nor the DOR-specific ligand [D-Pen2, Pen5]-enkephalin (DPDPE) had significant effects on food intake. However, when administered in combination with DAMGO, DPDPE significantly suppressed the magnitude of DAMGO-evoked feeding. Further analysis of DPDPE effects revealed that the drug strongly increased locomotor activity. Suppressive effects on feeding, then, may have occurred through competition between feeding and locomotion for behavioral expression.
Copyright 2009 Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
Convergent, not serial, striatal and pallidal circuits regulate opioid-induced food intake.Neuroscience. 2009 Jul 7;161(3):718-33. doi: 10.1016/j.neuroscience.2009.03.057. Epub 2009 Mar 29. Neuroscience. 2009. PMID: 19336249 Free PMC article.
-
Analysis of dopamine receptor antagonism upon feeding elicited by mu and delta opioid agonists in the shell region of the nucleus accumbens.Brain Res. 2000 Sep 15;877(1):65-72. doi: 10.1016/s0006-8993(00)02674-3. Brain Res. 2000. PMID: 10980244
-
Multiple opioid receptors mediate feeding elicited by mu and delta opioid receptor subtype agonists in the nucleus accumbens shell in rats.Brain Res. 2000 Sep 8;876(1-2):76-87. doi: 10.1016/s0006-8993(00)02631-7. Brain Res. 2000. PMID: 10973595
-
Amylin receptor signaling in the nucleus accumbens negatively modulates μ-opioid-driven feeding.Neuropsychopharmacology. 2014 Dec;39(13):3009-17. doi: 10.1038/npp.2014.153. Epub 2014 Jun 24. Neuropsychopharmacology. 2014. PMID: 24957819 Free PMC article.
-
From taste hedonics to motivational drive: central μ-opioid receptors and binge-eating behaviour.Int J Neuropsychopharmacol. 2009 Aug;12(7):995-1008. doi: 10.1017/S146114570900039X. Epub 2009 May 12. Int J Neuropsychopharmacol. 2009. PMID: 19433009 Review.
Cited by
-
Opioid hedonic hotspot in nucleus accumbens shell: mu, delta, and kappa maps for enhancement of sweetness "liking" and "wanting".J Neurosci. 2014 Mar 19;34(12):4239-50. doi: 10.1523/JNEUROSCI.4458-13.2014. J Neurosci. 2014. PMID: 24647944 Free PMC article.
-
Central effects of ethanol interact with endogenous mu-opioid activity to control isolation-induced analgesia in maternally separated infant rats.Behav Brain Res. 2014 Mar 1;260:119-30. doi: 10.1016/j.bbr.2013.11.043. Epub 2013 Dec 4. Behav Brain Res. 2014. PMID: 24315831 Free PMC article.
-
Pseudoginsenoside-F11 inhibits methamphetamine-induced behaviors by regulating dopaminergic and GABAergic neurons in the nucleus accumbens.Psychopharmacology (Berl). 2016 Mar;233(5):831-40. doi: 10.1007/s00213-015-4159-8. Epub 2015 Dec 1. Psychopharmacology (Berl). 2016. PMID: 26621348
-
Mu opioid receptors on vGluT2-expressing glutamatergic neurons modulate opioid reward.Addict Biol. 2021 May;26(3):e12942. doi: 10.1111/adb.12942. Epub 2020 Jul 20. Addict Biol. 2021. PMID: 32686251 Free PMC article.
-
Pharmacological traits of delta opioid receptors: pitfalls or opportunities?Psychopharmacology (Berl). 2013 Jul;228(1):1-18. doi: 10.1007/s00213-013-3129-2. Epub 2013 May 7. Psychopharmacology (Berl). 2013. PMID: 23649885 Free PMC article. Review.
References
-
- Bakshi VP, Kelley AE. Feeding induced by opioid stimulation of the ventral striatum: role of opiate receptor subtypes. J Pharmacol Exp Ther. 1993a;265:1253–1260. - PubMed
-
- Bakshi VP, Kelley AE. Striatal regulation of morphine-induced hyperphagia: an anatomical mapping study. Psychopharmacology (Berl) 1993b;111:207–214. - PubMed
-
- Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS. Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther. 1993;264:489–495. - PubMed
-
- Beczkowska IW, Koch JE, Bostock ME, Leibowitz SF, Bodnar RJ. Central opioid receptor subtype antagonists differentially reduce intake of saccharin and maltose dextrin solutions in rats. Brain Res. 1993;618:261–270. - PubMed
-
- Berridge KC. Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neurosci Biobehav Rev. 2000;24:173–198. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

