Control of directionality in the DNA strand-exchange reaction catalysed by the tyrosine recombinase TnpI
- PMID: 20044348
- PMCID: PMC2847244
- DOI: 10.1093/nar/gkp1187
Control of directionality in the DNA strand-exchange reaction catalysed by the tyrosine recombinase TnpI
Abstract
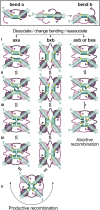
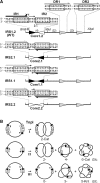
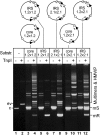
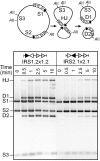
In DNA site-specific recombination catalysed by tyrosine recombinases, two pairs of DNA strands are sequentially exchanged between separate duplexes and the mechanisms that confer directionality to this theoretically reversible reaction remain unclear. The tyrosine recombinase TnpI acts at the internal resolution site (IRS) of the transposon Tn4430 to resolve intermolecular transposition products. Recombination is catalysed at the IRS core sites (IR1-IR2) and is regulated by adjacent TnpI-binding motifs (DR1 and DR2). These are dispensable accessory sequences that confer resolution selectivity to the reaction by stimulating synapsis between directly repeated IRSs. Here, we show that formation of the DR1-DR2-containing synapse imposes a specific order of activation of the TnpI catalytic subunits in the complex so that the IR1-bound subunits catalyse the first strand exchange and the IR2-bound subunits the second strand exchange. This ordered pathway was demonstrated for a complete recombination reaction using a TnpI catalytic mutant (TnpI-H234L) partially defective in DNA rejoining. The presence of the DR1- and DR2-bound TnpI subunits was also found to stabilize transient recombination intermediates, further displacing the reaction equilibrium towards product formation. Implication of TnpI/IRS accessory elements in the initial architecture of the synapse and subsequent conformational changes taking place during strand exchange is discussed.
Figures
Similar articles
-
Self-control in DNA site-specific recombination mediated by the tyrosine recombinase TnpI.Mol Microbiol. 2006 May;60(3):617-29. doi: 10.1111/j.1365-2958.2006.05127.x. Mol Microbiol. 2006. PMID: 16629665
-
TnpI recombinase: identification of sites within Tn5401 required for TnpI binding and site-specific recombination.J Bacteriol. 1995 Jul;177(14):4036-42. doi: 10.1128/jb.177.14.4036-4042.1995. J Bacteriol. 1995. PMID: 7608077 Free PMC article.
-
Multiple roles for TnpI recombinase in regulation of Tn5401 transposition in Bacillus thuringiensis.J Bacteriol. 1999 Oct;181(20):6271-7. doi: 10.1128/JB.181.20.6271-6277.1999. J Bacteriol. 1999. PMID: 10515914 Free PMC article.
-
Intermediates in serine recombinase-mediated site-specific recombination.Biochem Soc Trans. 2011 Apr;39(2):617-22. doi: 10.1042/BST0390617. Biochem Soc Trans. 2011. PMID: 21428950 Review.
-
Orchestrating serine resolvases.Biochem Soc Trans. 2010 Apr;38(2):384-7. doi: 10.1042/BST0380384. Biochem Soc Trans. 2010. PMID: 20298188 Free PMC article. Review.
Cited by
-
FtsK translocation permits discrimination between an endogenous and an imported Xer/dif recombination complex.Proc Natl Acad Sci U S A. 2016 Jul 12;113(28):7882-7. doi: 10.1073/pnas.1523178113. Epub 2016 Jun 17. Proc Natl Acad Sci U S A. 2016. PMID: 27317749 Free PMC article.
-
Xer Site Specific Recombination: Double and Single Recombinase Systems.Front Microbiol. 2017 Mar 20;8:453. doi: 10.3389/fmicb.2017.00453. eCollection 2017. Front Microbiol. 2017. PMID: 28373867 Free PMC article. Review.
References
-
- Craig N, Craigie R, Gellert M, Lambowitz A, editors. Mobile DNA II. Washington, DC: ASM Press; 2002.
-
- Hallet B, Sherratt DJ. Transposition and site-specific recombination: adapting DNA cut-and-paste mechanisms to a variety of genetic rearrangements. FEMS Microbiol. Rev. 1997;21:157–178. - PubMed
-
- Curcio MJ, Derbyshire KM. The outs and ins of transposition: from mu to kangaroo. Nat. Rev. Mol. Cell Biol. 2003;4:865–877. - PubMed
-
- Van Duyne GD. A structural view of cre-loxp site-specific recombination. Annu. Rev. Biophys. Biomol. Struct. 2001;30:87–104. - PubMed
-
- Grindley ND, Whiteson KL, Rice PA. Mechanisms of site-specific recombination. Annu. Rev. Biochem. 2006;75:567–605. - PubMed

