Regulation of acid signaling in rat pulmonary sensory neurons by protease-activated receptor-2
- PMID: 20044436
- PMCID: PMC2838668
- DOI: 10.1152/ajplung.00381.2009
Regulation of acid signaling in rat pulmonary sensory neurons by protease-activated receptor-2
Abstract
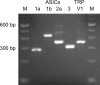
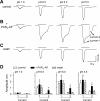
Airway acidification has been consistently observed in airway inflammatory conditions and is known to cause cardiorespiratory symptoms that are, at least in part, mediated through the activation of bronchopulmonary C fibers and the subsequent reflexes. Protease-activated receptor-2 (PAR(2)) is expressed in a variety of cells in the lung and airways and is believed to play a role in airway inflammation and hyperresponsiveness. This study was carried out to investigate the effect of PAR(2) activation on the acid signaling in rat bronchopulmonary C-fiber sensory neurons. Our RT-PCR results revealed the expression of mRNAs for transient receptor potential vanilloid receptor 1 (TRPV1) and four functional acid-sensing ion channel (ASIC) subunits 1a, 1b, 2a, and 3 in these sensory neurons. Preincubation of SLIGRL-NH(2), a specific PAR(2)-activating peptide, markedly enhanced the Ca(2+) transient evoked by extracellular acidification. Pretreatment with PAR(2) agonists significantly potentiated both acid-evoked ASIC- and TRPV1-like whole cell inward currents. Activation of PAR(2) also potentiated the excitability of these neurons to acid, but not electrical stimulation. In addition, the potentiation of acid-evoked responses was not prevented by inhibiting either PLC or PKC nor was mimicked by activation of PKC. In conclusion, activation of PAR(2) modulates the acid signaling in pulmonary sensory neurons, and the interaction may play a role in the pathogenesis of airway inflammatory conditions, where airway acidification and PAR(2) activation can occur simultaneously.
Figures

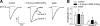
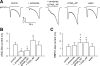


Similar articles
-
Protease-activated receptor-2 inhibits BK channel activity in bronchopulmonary sensory neurons.Neurosci Lett. 2015 Mar 4;589:13-8. doi: 10.1016/j.neulet.2015.01.020. Epub 2015 Jan 8. Neurosci Lett. 2015. PMID: 25578948
-
Differential regulation of ASICs and TRPV1 by zinc in rat bronchopulmonary sensory neurons.Lung. 2014 Dec;192(6):927-34. doi: 10.1007/s00408-014-9634-1. Epub 2014 Aug 10. Lung. 2014. PMID: 25108402
-
Activation of bitter taste receptors in pulmonary nociceptors sensitizes TRPV1 channels through the PLC and PKC signaling pathway.Am J Physiol Lung Cell Mol Physiol. 2017 Mar 1;312(3):L326-L333. doi: 10.1152/ajplung.00468.2016. Epub 2017 Jan 6. Am J Physiol Lung Cell Mol Physiol. 2017. PMID: 28062485
-
Interaction between TRPA1 and TRPV1: Synergy on pulmonary sensory nerves.Pulm Pharmacol Ther. 2015 Dec;35:87-93. doi: 10.1016/j.pupt.2015.08.003. Epub 2015 Aug 14. Pulm Pharmacol Ther. 2015. PMID: 26283426 Free PMC article. Review.
-
Acid-sensitive ion channels and receptors.Handb Exp Pharmacol. 2009;(194):283-332. doi: 10.1007/978-3-540-79090-7_9. Handb Exp Pharmacol. 2009. PMID: 19655111 Free PMC article. Review.
Cited by
-
Mechanisms underlying the stimulatory effect of inhaled sulfur dioxide on vagal bronchopulmonary C-fibres.J Physiol. 2020 Mar;598(5):1093-1108. doi: 10.1113/JP279152. Epub 2020 Feb 14. J Physiol. 2020. PMID: 31891193 Free PMC article.
-
Acid-sensing by airway afferent nerves.Pulm Pharmacol Ther. 2013 Oct;26(5):491-7. doi: 10.1016/j.pupt.2013.03.010. Epub 2013 Mar 21. Pulm Pharmacol Ther. 2013. PMID: 23524016 Free PMC article.
-
Infectious and Inflammatory Pathways to Cough.Annu Rev Physiol. 2023 Feb 10;85:71-91. doi: 10.1146/annurev-physiol-031422-092315. Epub 2022 Sep 28. Annu Rev Physiol. 2023. PMID: 36170660 Free PMC article. Review.
-
Airway smooth muscle in airway reactivity and remodeling: what have we learned?Am J Physiol Lung Cell Mol Physiol. 2013 Dec;305(12):L912-33. doi: 10.1152/ajplung.00259.2013. Epub 2013 Oct 18. Am J Physiol Lung Cell Mol Physiol. 2013. PMID: 24142517 Free PMC article. Review.
-
Regulation of particulate matter-induced mucin secretion by transient receptor potential vanilloid 1 receptors.Inflammation. 2012 Dec;35(6):1851-9. doi: 10.1007/s10753-012-9506-x. Inflammation. 2012. PMID: 22829138
References
-
- Amadesi S, Nie J, Vergnolle N, Cottrell GS, Grady EF, Trevisani M, Manni C, Geppetti P, McRoberts JA, Ennes H, Davis JB, Mayer EA, Bunnett NW. Protease-activated receptor 2 sensitizes the capsaicin receptor transient receptor potential vanilloid receptor 1 to induce hyperalgesia. J Neurosci 24: 4300–4312, 2004. - PMC - PubMed
-
- Asokananthan N, Graham PT, Stewart DJ, Bakker AJ, Eidne KA, Thompson PJ, Stewart GA. House dust mite allergens induce proinflammatory cytokines from respiratory epithelial cells: the cysteine protease allergen, Der p1, activates protease-activated receptor (PAR)-2 and inactivates PAR-1. J Immunol 169: 4572–4578, 2002 - PubMed
-
- Barrios VE, Jarosinski MA, Wright CD. Proteinase-activated receptor-2 mediates hyperresponsiveness in isolated guinea pig bronchi. Biochem Pharmacol 66: 519–525, 2003 - PubMed
-
- Benson CJ, Sutherland SP. Toward an understanding of the molecules that sense myocardial ischemia. Ann NY Acad Sci 940: 96–109, 2001 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

