A gain-of-function mutation of Arabidopsis lipid transfer protein 5 disturbs pollen tube tip growth and fertilization
- PMID: 20044438
- PMCID: PMC2814499
- DOI: 10.1105/tpc.109.070854
A gain-of-function mutation of Arabidopsis lipid transfer protein 5 disturbs pollen tube tip growth and fertilization
Abstract
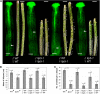
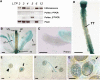
During compatible pollination of the angiosperms, pollen tubes grow in the pistil transmitting tract (TT) and are guided to the ovule for fertilization. Lily (Lilium longiflorum) stigma/style Cys-rich adhesin (SCA), a plant lipid transfer protein (LTP), is a small, secreted peptide involved in pollen tube adhesion-mediated guidance. Here, we used a reverse genetic approach to study biological roles of Arabidopsis thaliana LTP5, a SCA-like LTP. The T-DNA insertional gain-of-function mutant plant for LTP5 (ltp5-1) exhibited ballooned pollen tubes, delayed pollen tube growth, and decreased numbers of fertilized eggs. Our reciprocal cross-pollination study revealed that ltp5-1 results in both male and female partial sterility. RT-PCR and beta-glucuronidase analyses showed that LTP5 is present in pollen and the pistil TT in low levels. Pollen-targeted overexpression of either ltp5-1 or wild-type LTP5 resulted in defects in polar tip growth of pollen tubes and thereby decreased seed set, suggesting that mutant ltp5-1 acts as a dominant-active form of wild-type LTP5 in pollen tube growth. The ltp5-1 protein has additional hydrophobic C-terminal sequences, compared with LTP5. In our structural homology/molecular dynamics modeling, Tyr-91 in ltp5-1, replacing Val-91 in LTP5, was predicted to interact with Arg-45 and Tyr-81, which are known to interact with a lipid ligand in maize (Zea mays) LTP. Thus, Arabidopsis LTP5 plays a significant role in reproduction.
Figures





Similar articles
-
A multifaceted study of stigma/style cysteine-rich adhesin (SCA)-like Arabidopsis lipid transfer proteins (LTPs) suggests diversified roles for these LTPs in plant growth and reproduction.J Exp Bot. 2010 Oct;61(15):4277-90. doi: 10.1093/jxb/erq228. Epub 2010 Jul 28. J Exp Bot. 2010. PMID: 20667964 Free PMC article.
-
Pollen tube growth and guidance: roles of small, secreted proteins.Ann Bot. 2011 Sep;108(4):627-36. doi: 10.1093/aob/mcr015. Epub 2011 Feb 8. Ann Bot. 2011. PMID: 21307038 Free PMC article. Review.
-
A Putative Protein O-Fucosyltransferase Facilitates Pollen Tube Penetration through the Stigma-Style Interface.Plant Physiol. 2018 Apr;176(4):2804-2818. doi: 10.1104/pp.17.01577. Epub 2018 Feb 21. Plant Physiol. 2018. PMID: 29467178 Free PMC article.
-
Arabidopsis HAP2 (GCS1) is a sperm-specific gene required for pollen tube guidance and fertilization.Development. 2006 Dec;133(23):4761-9. doi: 10.1242/dev.02683. Epub 2006 Nov 1. Development. 2006. PMID: 17079265
-
Functional genomics of pollen tube-pistil interactions in Arabidopsis.Biochem Soc Trans. 2010 Apr;38(2):593-7. doi: 10.1042/BST0380593. Biochem Soc Trans. 2010. PMID: 20298227 Review.
Cited by
-
Screening of Key Proteins Affecting Floral Initiation of Saffron Under Cold Stress Using iTRAQ-Based Proteomics.Front Plant Sci. 2021 May 11;12:644934. doi: 10.3389/fpls.2021.644934. eCollection 2021. Front Plant Sci. 2021. PMID: 34046047 Free PMC article.
-
The non-specific lipid transfer protein N5 of Medicago truncatula is implicated in epidermal stages of rhizobium-host interaction.BMC Plant Biol. 2012 Dec 7;12:233. doi: 10.1186/1471-2229-12-233. BMC Plant Biol. 2012. PMID: 23217154 Free PMC article.
-
LTP3 contributes to disease susceptibility in Arabidopsis by enhancing abscisic acid (ABA) biosynthesis.Mol Plant Pathol. 2016 Apr;17(3):412-26. doi: 10.1111/mpp.12290. Epub 2015 Jul 30. Mol Plant Pathol. 2016. PMID: 26123657 Free PMC article.
-
Transcript Analysis and Regulative Events during Flower Development in Olive (Olea europaea L.).PLoS One. 2016 Apr 14;11(4):e0152943. doi: 10.1371/journal.pone.0152943. eCollection 2016. PLoS One. 2016. PMID: 27077738 Free PMC article.
-
On the role of a Lipid-Transfer Protein. Arabidopsis ltp3 mutant is compromised in germination and seedling growth.Plant Signal Behav. 2015;10(12):e1105417. doi: 10.1080/15592324.2015.1105417. Plant Signal Behav. 2015. PMID: 26479260 Free PMC article.
References
-
- Abramoff, M.D., Magelhaes, P.J., and Ram, S.J. (2004). Image processing with ImageJ. Biophotonics International 11 36–42.
-
- Alonso, J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301 653–657. - PubMed
-
- Altschul, S.F., Gish, W., Miller, W., Myers, E.W., and Lipman, D.J. (1990). Basic local alignment search tool. J. Mol. Biol. 215 403–410. - PubMed
-
- Arondel, V., Vergnolle, C., Cantrel, C., and Kader, J.C. (2000). Lipid transfer proteins are encoded by a small multigene family in Arabidopsis thaliana. Plant Sci. 157 1–12. - PubMed
-
- Baker, K.A., Moore, S.W., Jarjour, A.A., and Kennedy, T.E. (2006). When a diffusible axon guidance cue stops diffusing: roles for netrins in adhesion and morphogenesis. Curr. Opin. Neurobiol. 16 529–534. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

