Identification and functional characterization of monofunctional ent-copalyl diphosphate and ent-kaurene synthases in white spruce reveal different patterns for diterpene synthase evolution for primary and secondary metabolism in gymnosperms
- PMID: 20044448
- PMCID: PMC2832265
- DOI: 10.1104/pp.109.151456
Identification and functional characterization of monofunctional ent-copalyl diphosphate and ent-kaurene synthases in white spruce reveal different patterns for diterpene synthase evolution for primary and secondary metabolism in gymnosperms
Abstract
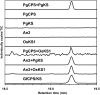
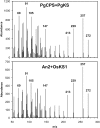
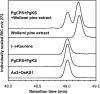
The biosynthesis of the tetracyclic diterpene ent-kaurene is a critical step in the general (primary) metabolism of gibberellin hormones. ent-Kaurene is formed by a two-step cyclization of geranylgeranyl diphosphate via the intermediate ent-copalyl diphosphate. In a lower land plant, the moss Physcomitrella patens, a single bifunctional diterpene synthase (diTPS) catalyzes both steps. In contrast, in angiosperms, the two consecutive cyclizations are catalyzed by two distinct monofunctional enzymes, ent-copalyl diphosphate synthase (CPS) and ent-kaurene synthase (KS). The enzyme, or enzymes, responsible for ent-kaurene biosynthesis in gymnosperms has been elusive. However, several bifunctional diTPS of specialized (secondary) metabolism have previously been characterized in gymnosperms, and all known diTPSs for resin acid biosynthesis in conifers are bifunctional. To further understand the evolution of ent-kaurene biosynthesis as well as the evolution of general and specialized diterpenoid metabolisms in gymnosperms, we set out to determine whether conifers use a single bifunctional diTPS or two monofunctional diTPSs in the ent-kaurene pathway. Using a combination of expressed sequence tag, full-length cDNA, genomic DNA, and targeted bacterial artificial chromosome sequencing, we identified two candidate CPS and KS genes from white spruce (Picea glauca) and their orthologs in Sitka spruce (Picea sitchensis). Functional characterization of the recombinant enzymes established that ent-kaurene biosynthesis in white spruce is catalyzed by two monofunctional diTPSs, PgCPS and PgKS. Comparative analysis of gene structures and enzyme functions highlights the molecular evolution of these diTPSs as conserved between gymnosperms and angiosperms. In contrast, diTPSs for specialized metabolism have evolved differently in angiosperms and gymnosperms.
Figures





Similar articles
-
Mutational analysis of white spruce (Picea glauca) ent-kaurene synthase (PgKS) reveals common and distinct mechanisms of conifer diterpene synthases of general and specialized metabolism.Phytochemistry. 2012 Feb;74:30-9. doi: 10.1016/j.phytochem.2011.11.004. Epub 2011 Dec 15. Phytochemistry. 2012. PMID: 22177479
-
Divergent Evolution of the Diterpene Biosynthesis Pathway in Tea Plants (Camellia sinensis) Caused by Single Amino Acid Variation of ent-Kaurene Synthase.J Agric Food Chem. 2020 Sep 16;68(37):9930-9939. doi: 10.1021/acs.jafc.0c03488. Epub 2020 Sep 3. J Agric Food Chem. 2020. PMID: 32841021
-
Ent-kaurene synthase from the fungus Phaeosphaeria sp. L487. cDNA isolation, characterization, and bacterial expression of a bifunctional diterpene cyclase in fungal gibberellin biosynthesis.J Biol Chem. 1997 Aug 29;272(35):21706-12. doi: 10.1074/jbc.272.35.21706. J Biol Chem. 1997. PMID: 9268298
-
Evolution of Labdane-Related Diterpene Synthases in Cereals.Plant Cell Physiol. 2020 Dec 23;61(11):1850-1859. doi: 10.1093/pcp/pcaa106. Plant Cell Physiol. 2020. PMID: 32810270 Review.
-
The family of terpene synthases in plants: a mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom.Plant J. 2011 Apr;66(1):212-29. doi: 10.1111/j.1365-313X.2011.04520.x. Plant J. 2011. PMID: 21443633 Review.
Cited by
-
Evolution of conifer diterpene synthases: diterpene resin acid biosynthesis in lodgepole pine and jack pine involves monofunctional and bifunctional diterpene synthases.Plant Physiol. 2013 Feb;161(2):600-16. doi: 10.1104/pp.112.208546. Epub 2012 Dec 12. Plant Physiol. 2013. PMID: 23370714 Free PMC article.
-
The Pinus taeda genome is characterized by diverse and highly diverged repetitive sequences.BMC Genomics. 2010 Jul 7;11:420. doi: 10.1186/1471-2164-11-420. BMC Genomics. 2010. PMID: 20609256 Free PMC article.
-
Domain loss has independently occurred multiple times in plant terpene synthase evolution.Plant J. 2011 Dec;68(6):1051-60. doi: 10.1111/j.1365-313X.2011.04756.x. Epub 2011 Oct 17. Plant J. 2011. PMID: 21999670 Free PMC article.
-
Manoyl oxide (13R), the biosynthetic precursor of forskolin, is synthesized in specialized root cork cells in Coleus forskohlii.Plant Physiol. 2014 Mar;164(3):1222-36. doi: 10.1104/pp.113.228429. Epub 2014 Jan 30. Plant Physiol. 2014. PMID: 24481136 Free PMC article.
-
Sequence-Structure Analysis Unlocking the Potential Functional Application of the Local 3D Motifs of Plant-Derived Diterpene Synthases.Biomolecules. 2024 Jan 17;14(1):120. doi: 10.3390/biom14010120. Biomolecules. 2024. PMID: 38254720 Free PMC article.
References
-
- Adams RP. (2007) Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, Ed 4. Allured Publishing, Carol Stream, IL
-
- Anterola A, Shanle E. (2008) Genomic insights in moss gibberellin biosynthesis. Bryologist 111: 218–230
-
- Aubourg S, Lecharny A, Bohlmann J. (2002) Genomic analysis of the terpenoid synthase (AtTPS) gene family of Arabidopsis thaliana. Mol Genet Genomics 267: 730–745 - PubMed
-
- Bohlmann J. (2008) Insect-induced terpenoid defenses in spruce. Schaller A, , Induced Plant Resistance to Herbivory. Springer Science, New York, 173–187
-
- Bohlmann J, Keeling CI. (2008) Terpenoid biomaterials. Plant J 54: 656–669 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

