Intracellular calreticulin regulates multiple steps in fibrillar collagen expression, trafficking, and processing into the extracellular matrix
- PMID: 20044481
- PMCID: PMC2844156
- DOI: 10.1074/jbc.M109.006841
Intracellular calreticulin regulates multiple steps in fibrillar collagen expression, trafficking, and processing into the extracellular matrix
Abstract
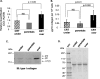
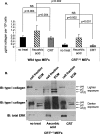
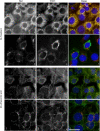
Calreticulin (CRT), a chaperone and Ca(2+) regulator, enhances wound healing, and its expression correlates with fibrosis in animal models, suggesting that CRT regulates production of the extracellular matrix. However, direct regulation of collagen matrix by CRT has not been previously demonstrated. We investigated the role of CRT in the regulation of fibrillar collagen expression, secretion, processing, and deposition in the extracellular matrix by fibroblasts. Mouse embryonic fibroblasts deficient in CRT (CRT(-/-) MEFs) have reduced transcript levels of fibrillar collagen I and III and less soluble collagen as compared with wild type MEFs. Correspondingly, fibroblasts engineered to overexpress CRT have increased collagen type I transcript and protein. Collagen expression appears to be regulated by endoplasmic reticulum (ER) calcium levels and intracellular CRT, because thapsigargin treatment reduced collagen expression, whereas addition of exogenous recombinant CRT had no effect. CRT(-/-) MEFs exhibited increased ER retention of collagen, and collagen and CRT were co-immunoprecipitated from isolated cell lysates, suggesting that CRT is important for trafficking of collagen through the ER. CRT(-/-) MEFs also have reduced type I procollagen processing and deposition into the extracellular matrix. The reduced collagen matrix deposition is partly a consequence of reduced fibronectin matrix formation in the CRT-deficient cells. Together, these data show that CRT complexes with collagen in cells and that CRT plays critical roles at multiple stages of collagen expression and processing. These data identify CRT as an important regulator of collagen and suggest that intracellular CRT signaling plays an important role in tissue remodeling and fibrosis.
Figures








References
-
- Opas M., Dziak E., Fliegel L., Michalak M. (1991) J. Cell Physiol. 149, 160–171 - PubMed
-
- Eggleton P., Lieu T. S., Zappi E. G., Sastry K., Coburn J., Zaner K. S., Sontheimer R. D., Capra J. D., Ghebrehiwet B., Tauber A. I. (1994) Clin. Immunol. Immunopathol. 72, 405–409 - PubMed
-
- Gray A. J., Park P. W., Broekelmann T. J., Laurent G. J., Reeves J. T., Stenmark K. R., Mecham R. P. (1995) J. Biol. Chem. 270, 26602–26606 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

