The C5a receptor (C5aR) C5L2 is a modulator of C5aR-mediated signal transduction
- PMID: 20044484
- PMCID: PMC2844210
- DOI: 10.1074/jbc.M109.092106
The C5a receptor (C5aR) C5L2 is a modulator of C5aR-mediated signal transduction
Abstract
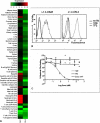
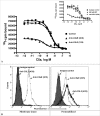
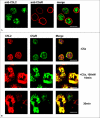
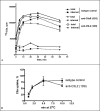
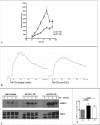
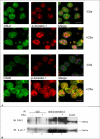
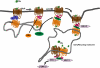
The complement anaphylatoxin C5a is a proinflammatory component of host defense that functions through two identified receptors, C5a receptor (C5aR) and C5L2. C5aR is a classical G protein-coupled receptor, whereas C5L2 is structurally homologous but deficient in G protein coupling. In human neutrophils, we show C5L2 is predominantly intracellular, whereas C5aR is expressed on the plasma membrane. Confocal analysis shows internalized C5aR following ligand binding is co-localized with both C5L2 and beta-arrestin. Antibody blockade of C5L2 results in a dramatic increase in C5a-mediated chemotaxis and ERK1/2 phosphorylation but does not alter C5a-mediated calcium mobilization, supporting its role in modulation of the beta-arrestin pathway. Association of C5L2 with beta-arrestin is confirmed by cellular co-immunoprecipitation assays. C5L2 blockade also has no effect on ligand uptake or C5aR endocytosis in human polymorphonuclear leukocytes, distinguishing its role from that of a rapid recycling or scavenging receptor in this cell type. This is thus the first example of a naturally occurring seven-transmembrane segment receptor that is both obligately uncoupled from G proteins and a negative modulator of signal transduction through the beta-arrestin pathway. Physiologically, these properties provide the possibility for additional fine-tuning of host defense.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

