The effect of 17 beta-estradiol on intracellular calcium homeostasis in human endothelial cells
- PMID: 20044991
- PMCID: PMC2822064
- DOI: 10.1016/j.ejphar.2009.12.030
The effect of 17 beta-estradiol on intracellular calcium homeostasis in human endothelial cells
Abstract
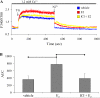
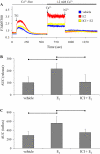
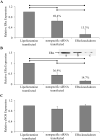
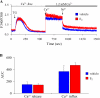
The cardiovascular effects of estrogen are mediated in part by augmenting the function of endothelial nitric oxide synthase. Endothelial nitric oxide synthase activity is dependent on many cofactors including Ca(2+). Hence, we investigated the effect of chronic 17 beta-estradiol treatment on the intracellular Ca(2+) concentration and endothelial nitric oxide synthase protein expression in the human endothelial cell line, EA.hy926, using spectrofluorometry and Western blot, respectively. Inhibiting the sarco(endo)plasmic reticulum Ca(2+) ATPase with thapsigargin caused an increase in the intracellular Ca(2+) concentration, which was higher in chronically 17 beta-estradiol-treated (1muM, 24h) cells loaded with Fura-2-acetoxymethyl ester compared to vehicle-treated cells, suggesting a higher endoplasmic reticulum Ca(2+) content in 17 beta-estradiol-treated cells. An enhanced Ca(2+) influx pathway in chronically 17 beta-estradiol-treated cells was also observed. In addition, 17 beta-estradiol-treated cells expressed higher levels of endothelial nitric oxide synthase protein in comparison to vehicle-treated cells. The chronic effect of 17 beta-estradiol on Ca(2+) homeostasis and endothelial nitric oxide synthase expression was attenuated with the nonselective estrogen receptor inhibitor, ICI 182,780 (10muM, 7alpha, 17beta-[9-[(4,4,5,5,5-Pentafluoropentyl)sulfinyl]nonyl] estra-1,3,5(10)-triene-3,17-diol). Furthermore, analysis of the thapsigargin-evoked Ca(2+) response in chronically 17 beta-estradiol-treated estrogen receptor alpha-knockdown cells showed no significant difference in Ca(2+) response compared to vehicle-treated estrogen receptor alpha-knockdown cells, indicating that the regulation of Ca(2+) homeostasis by 17 beta-estradiol is mediated through an estrogen receptor alpha-dependent pathway. These data revealed an estrogen receptor alpha-dependent modulation of Ca(2+) homeostasis accompanying the enhancement of endothelial nitric oxide synthase expression in 17 beta-estradiol-treated human endothelial cells.
Figures
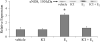

References
-
- Barrett-Connor E, Bush TL. Estrogen and coronary heart disease in women. JAMA. 1991;265:1861–1867. - PubMed
-
- Bryan RM, Jr., You J, Golding EM, Marrelli SP. Endothelium-derived hyperpolarizing factor: a cousin to nitric oxide and prostacyclin. Anesthesiology. 2005;102:1261–1277. - PubMed
-
- Busse R, Edwards G, Feletou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: bringing the concepts together. Trends Pharmacol Sci. 2002;23:374–380. - PubMed
-
- Deenadayalu VP, White RE, Stallone JN, Gao X, Garcia AJ. Testosterone relaxes coronary arteries by opening the large-conductance, calcium-activated potassium channel. Am J Physiol Heart Circ Physiol. 2001;281:H1720–1727. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

