Modulation of astrocyte glutamate transporters decreases seizures in a mouse model of Tuberous Sclerosis Complex
- PMID: 20045054
- PMCID: PMC2823985
- DOI: 10.1016/j.nbd.2009.12.020
Modulation of astrocyte glutamate transporters decreases seizures in a mouse model of Tuberous Sclerosis Complex
Abstract
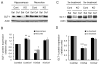
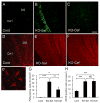
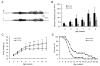
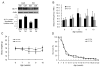
Astrocyte dysfunction may contribute to epileptogenesis and other neurological deficits in Tuberous Sclerosis Complex (TSC). In particular, decreased expression and function of astrocyte glutamate transporters have been implicated in causing elevated extracellular glutamate levels, neuronal death, and epilepsy in a mouse model of TSC (Tsc1(GFAP)CKO mice), involving inactivation of the Tsc1 gene primarily in astrocytes. Here, we tested whether pharmacological induction of astrocyte glutamate transporter expression can prevent the neurological phenotype of Tsc1(GFAP)CKO mice. Early treatment with ceftriaxone prior to the onset of epilepsy increased expression of astrocyte glutamate transporters, decreased extracellular glutamate levels, neuronal death, and seizure frequency, and improved survival in Tsc1(GFAP)CKO mice. In contrast, late treatment with ceftriaxone after onset of epilepsy increased glutamate transporter expression, but had no effect on seizures. These results indicate that astrocyte glutamate transporters contribute to epileptogenesis in Tsc1(GFAP)CKO mice and suggest novel therapeutic strategies for epilepsy in TSC directed at astrocytes.
2009 Elsevier Inc. All rights reserved.
Figures
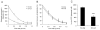
References
-
- Akbar MT, Rattray M, Williams RJ, Chong NW, Meldrum BS. Reduction of GABA and glutamate transporter messenger RNAs in the severe-seizure genetically epilepsy-prone rat. Neurosci. 1998;85:1235–1251. - PubMed
-
- Chu K, Lee ST, Sinn DI, Ko SY, Kim EH, Kim JM, Kim SJ, Park DK, Jung KH, Song EC, Lee SK, Kim M, Roh JK. Pharmacological induction of ischemic tolerance by glutamate transporter-1 (EAAT2) upregulation. Stroke. 2007;38:177–82. - PubMed
-
- Cirrito JR, May PC, O'Dell MA, Taylor JW, Parsadanian M, Cramer JW, Audia JE, Nissen JS, Bales KR, Paul SM, DeMattos RB, Holtzman DM. In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-β metabolism and half-life. J Neurosci. 2003;23:8844–8853. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

