One time intranasal vaccination with a modified vaccinia Tiantan strain MVTT(ZCI) protects animals against pathogenic viral challenge
- PMID: 20045097
- PMCID: PMC7127290
- DOI: 10.1016/j.vaccine.2009.12.038
One time intranasal vaccination with a modified vaccinia Tiantan strain MVTT(ZCI) protects animals against pathogenic viral challenge
Abstract
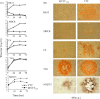
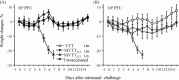
To combat variola virus in bioterrorist attacks, it is desirable to develop a noninvasive vaccine. Based on the vaccinia Tiantan (VTT) strain, which was historically used to eradicate the smallpox in China, we generated a modified VTT (MVTT(ZCI)) by removing the hemagglutinin gene and an 11,944bp genomic region from HindIII fragment C2L to F3L. MVTT(ZCI) was characterized for its host cell range in vitro and preclinical safety and efficacy profiles in mice. Despite replication-competency in some cell lines, unlike VTT, MVTT(ZCI) did not cause death after intracranial injection or body weight loss after intranasal inoculation. MVTT(ZCI) did not replicate in mouse brain and was safe in immunodeficient mice. MVTT(ZCI) induced neutralizing antibodies via the intranasal route of immunization. One time intranasal immunization protected animals from the challenge of the pathogenic vaccinia WR strain. This study established proof-of-concept that the attenuated replicating MVTT(ZCI) may serve as a safe noninvasive smallpox vaccine candidate.
Copyright 2009 Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
Mucosal immunization induces a higher level of lasting neutralizing antibody response in mice by a replication-competent smallpox vaccine: vaccinia Tiantan strain.J Biomed Biotechnol. 2011;2011:970424. doi: 10.1155/2011/970424. Epub 2011 Jun 20. J Biomed Biotechnol. 2011. PMID: 21765641 Free PMC article.
-
Generation of an attenuated Tiantan vaccinia virus by deletion of the ribonucleotide reductase large subunit.Arch Virol. 2014 Sep;159(9):2223-31. doi: 10.1007/s00705-014-2057-8. Epub 2014 Mar 28. Arch Virol. 2014. PMID: 24677065
-
A novel replication-competent vaccinia vector MVTT is superior to MVA for inducing high levels of neutralizing antibody via mucosal vaccination.PLoS One. 2009;4(1):e4180. doi: 10.1371/journal.pone.0004180. Epub 2009 Jan 13. PLoS One. 2009. PMID: 19159014 Free PMC article.
-
Clinical development of Modified Vaccinia virus Ankara vaccines.Vaccine. 2013 Sep 6;31(39):4241-6. doi: 10.1016/j.vaccine.2013.03.020. Epub 2013 Mar 21. Vaccine. 2013. PMID: 23523410 Review.
-
[Oral and nasal immunization with Poxvirus vacciniae. II. New methods of smallpox vaccination].Zentralbl Bakteriol Orig B. 1972 Aug;156(1):15-29. Zentralbl Bakteriol Orig B. 1972. PMID: 4575332 Review. German. No abstract available.
Cited by
-
Introduction of the six major genomic deletions of modified vaccinia virus Ankara (MVA) into the parental vaccinia virus is not sufficient to reproduce an MVA-like phenotype in cell culture and in mice.J Virol. 2010 Oct;84(19):9907-19. doi: 10.1128/JVI.00756-10. Epub 2010 Jul 28. J Virol. 2010. PMID: 20668072 Free PMC article.
-
Attenuation of vaccinia Tian Tan strain by removal of viral TC7L-TK2L and TA35R genes.PLoS One. 2012;7(2):e31979. doi: 10.1371/journal.pone.0031979. Epub 2012 Feb 21. PLoS One. 2012. PMID: 22363781 Free PMC article.
-
Generation of an Attenuated Tiantan Vaccinia Virus Strain by Deletion of Multiple Genes.Front Cell Infect Microbiol. 2017 Oct 31;7:462. doi: 10.3389/fcimb.2017.00462. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 29164070 Free PMC article.
-
Mucosal immunization induces a higher level of lasting neutralizing antibody response in mice by a replication-competent smallpox vaccine: vaccinia Tiantan strain.J Biomed Biotechnol. 2011;2011:970424. doi: 10.1155/2011/970424. Epub 2011 Jun 20. J Biomed Biotechnol. 2011. PMID: 21765641 Free PMC article.
-
Genomic sequence and virulence of clonal isolates of vaccinia virus Tiantan, the Chinese smallpox vaccine strain.PLoS One. 2013 Apr 12;8(4):e60557. doi: 10.1371/journal.pone.0060557. Print 2013. PLoS One. 2013. PMID: 23593246 Free PMC article.
References
-
- Fulginiti V.A., Papier A., Lane J.M., Neff J.M., Henderson D.A. Smallpox vaccination: a review, part II adverse events. Clin Infect Dis. 2003;37(July (2)):251–271. - PubMed
-
- Nafziger S.D. Smallpox. Crit Care Clin. 2005;21(October (4)):739–746. vii. - PubMed
-
- Berche P. The threat of smallpox and bioterrorism. Trends Microbiol. 2001;9(January (1)):15–18. - PubMed
-
- Drazen J.M. Smallpox and bioterrorism. N Engl J Med. 2002;346(April (17)):1262–1263. - PubMed
-
- Eckart R.E., Shry E.A., Atwood J.E., Brundage J.F., Lay J.C., Bateson T.F. Smallpox vaccination and ischemic coronary events in healthy adults. Vaccine. 2007;25(December (50)):8359–8364. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

