Validation and application of a liquid chromatography-tandem mass spectrometric method for quantification of the drug transport probe fexofenadine in human plasma using 96-well filter plates
- PMID: 20045385
- PMCID: PMC2818817
- DOI: 10.1016/j.jchromb.2009.12.022
Validation and application of a liquid chromatography-tandem mass spectrometric method for quantification of the drug transport probe fexofenadine in human plasma using 96-well filter plates
Abstract
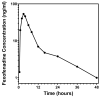
A rapid method to determine fexofenadine concentrations in human plasma using protein precipitation in 96-well plates and liquid chromatography-tandem mass spectrometry was validated. Plasma proteins were precipitated with acetonitrile containing the internal standard fexofenadine-d6, mixed briefly, and then filtered into a collection plate. The resulting filtrate was diluted and injected onto a Phenomenex Gemini C18 (50 mm x 2.0 mm, 5 microm) analytical column. The mobile phase consisted of 0.1% formic acid, 5 mM ammonium acetate in deionized water and methanol (35:65, v/v). The flow rate was 0.2 ml/min and the total run time was 2 min. Detection of the analytes was achieved using positive ion electrospray ionization and high resolution multiple reaction monitoring mode (H-SRM). The linear standard curve ranged from 1 to 500 ng/ml and the precision and accuracy (intra- and inter-run) were within 4.3% and 8.0%, respectively. The method has been applied successfully to determine fexofenadine concentrations in human plasma samples obtained from subjects administered a single oral dose of fexofenadine. The method is rapid, sensitive, selective and directly applicable to human pharmacokinetic studies involving fexofenadine.
2009 Elsevier B.V. All rights reserved.
Figures


References
-
- Devillier P, Roche N, Faisy C. Clin Pharmacokinet. 2008;47:217. - PubMed
-
- Lippert C, Ling J, Brown P, Burmaster S, Eller M, Cheng L. Pharm Res. 1995;12:S390.
-
- Cvetkovic M, Leake B, Fromm MF, Wilkinson GR, Kim RB. Drug Metab Dispos. 1999;27:866. - PubMed
-
- Matsushima S, Maeda K, Hayashi H, Debori Y, Schinkel AH, Schuetz JD, Kusuhara H, Sugiyama Y. Mol Pharmacol. 2008;73:1474. - PubMed
-
- Shimizu M, Fuse K, Okudaira K, Nishigaki R, Maeda K, Kusuhara H, Sugiyama Y. Drug Metab Dispos. 2005;33:1477. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

