Low level exposure to monomethyl arsonous acid-induced the over-production of inflammation-related cytokines and the activation of cell signals associated with tumor progression in a urothelial cell model
- PMID: 20045430
- PMCID: PMC2846965
- DOI: 10.1016/j.taap.2009.12.029
Low level exposure to monomethyl arsonous acid-induced the over-production of inflammation-related cytokines and the activation of cell signals associated with tumor progression in a urothelial cell model
Abstract
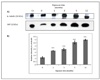
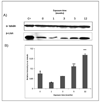
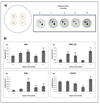
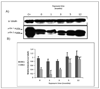
Human bladder cancer has been associated with chronic exposure to arsenic. Chronic exposure of an immortalized non-tumorigenic urothelial cell line (UROtsa cells) to arsenicals has transformed these cells to a malignant phenotype, but the involved mechanisms are not fully understood. Chronic inflammation has been linked with cancer development mainly because many pro-inflammatory cytokines, growth factors as well as angiogenic chemokines have been found in tumors. In this study the chronology of inflammatory cytokines production was profiled in UROtsa cells chronically exposed to the toxic arsenic metabolite, monomethylarsonous acid [50 nM MMA(III)] to know the role of inflammation in cell transformation. Acute 50 nM MMA(III) exposure induced over-production of many pro-inflammatory cytokines as soon as 12 h after acute exposure. The same cytokines remain over-regulated after chronic exposure to 50 nM MMA(III), especially after 3 mo exposure. At 3 mo exposure the sustained production of cytokines like IL-1, IL-6, IL-8 and TNF is coincident with the appearance of characteristics associated with cell transformation seen in other arsenic-UROtsa studies. The sustained and increased activation of NFkappaB and c-Jun is also present along the transformation process and the phosphorylated proteins p38 MAPK and ERK 1/2 are increased also through the time line. Taken together these results support the notion that chronic inflammation is associated within MMA(III)-induced cell transformation and may act as a promoting factor in UROtsa cell transformation.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures





Similar articles
-
Interleukin-8 (IL-8) over-production and autocrine cell activation are key factors in monomethylarsonous acid [MMA(III)]-induced malignant transformation of urothelial cells.Toxicol Appl Pharmacol. 2012 Jan 1;258(1):10-8. doi: 10.1016/j.taap.2011.10.002. Epub 2011 Oct 10. Toxicol Appl Pharmacol. 2012. PMID: 22015448 Free PMC article.
-
Global gene expression changes in human urothelial cells exposed to low-level monomethylarsonous acid.Toxicology. 2012 Jan 27;291(1-3):102-12. doi: 10.1016/j.tox.2011.11.002. Epub 2011 Nov 17. Toxicology. 2012. PMID: 22108045 Free PMC article.
-
Mitogenic signal transduction caused by monomethylarsonous acid in human bladder cells: role in arsenic-induced carcinogenesis.Toxicol Sci. 2007 Feb;95(2):321-30. doi: 10.1093/toxsci/kfl160. Epub 2006 Nov 8. Toxicol Sci. 2007. PMID: 17093206
-
Immortalized human urothelial cells as a model of arsenic-induced bladder cancer.Toxicology. 2008 Jun 27;248(2-3):67-76. doi: 10.1016/j.tox.2008.03.020. Epub 2008 Mar 30. Toxicology. 2008. PMID: 18456381 Review.
-
Sleeping beauty: awakening urothelium from its slumber.Am J Physiol Renal Physiol. 2017 Apr 1;312(4):F732-F743. doi: 10.1152/ajprenal.00337.2016. Epub 2017 Jan 25. Am J Physiol Renal Physiol. 2017. PMID: 28122714 Free PMC article. Review.
Cited by
-
Melatonin: a pleiotropic hormone as a novel potent therapeutic candidate in arsenic toxicity.Mol Biol Rep. 2021 Sep;48(9):6603-6618. doi: 10.1007/s11033-021-06669-3. Epub 2021 Aug 28. Mol Biol Rep. 2021. PMID: 34453671 Review.
-
Mechanisms by which interleukin-6 attenuates cell invasion and tumorigenesis in human bladder carcinoma cells.Biomed Res Int. 2013;2013:791212. doi: 10.1155/2013/791212. Epub 2013 May 16. Biomed Res Int. 2013. PMID: 23762858 Free PMC article.
-
Arsenite activates NFκB through induction of C-reactive protein.Toxicol Appl Pharmacol. 2012 Jun 15;261(3):263-70. doi: 10.1016/j.taap.2012.04.005. Epub 2012 Apr 12. Toxicol Appl Pharmacol. 2012. PMID: 22521605 Free PMC article.
-
ATF2 partly mediated the expressions of proliferative factors and inhibited pro-inflammatory factors' secretion in arsenite-treated human uroepithelial cells.Toxicol Res (Camb). 2017 Mar 29;6(4):468-476. doi: 10.1039/c6tx00407e. eCollection 2017 Jul 1. Toxicol Res (Camb). 2017. PMID: 30090515 Free PMC article.
-
BCG response prediction with cytokine gene variants and bladder cancer: where we are?J Cancer Res Clin Oncol. 2011 Dec;137(12):1729-38. doi: 10.1007/s00432-011-1056-3. Epub 2011 Sep 20. J Cancer Res Clin Oncol. 2011. PMID: 21932129 Free PMC article. Review.
References
-
- Aguirre-Bañuelos P, Escudero-Lourdes C, Sanchez-Peña LC, Del Razo LM, Perez-Urizar JT. Inorganic arsenic exposure affects pain behavior and inflammatory response in rat. Toxicol Appl Pharmacol. 2008;229(3):374–85. 15. - PubMed
-
- American Cancer Society. Cancer Facts and Figures 2009. Atlanta, (GA): American Cancer Society; 2009.
-
- Ariztia EV, Lee CJ, Gogoi R, Fishman DA. The tumor microenvironment: key to early detection. Crit Rev Clin Lab Sci. 2006;43(5–6):393–425. - PubMed
-
- Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Cogliano V. Carcinogenicity of some aromatic amines, organic dyes, and related exposures. Lancet Oncol. 2008;9(4):322–3. - PubMed
-
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–45. 17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

